Current Endocrine Therapy in Hormone-Receptor-Positive Breast Cancer: From Tumor Biology to the Rationale for Therapeutic Tunning
- PMID: 40731911
- PMCID: PMC12299218
- DOI: 10.3390/medicina61071280
Current Endocrine Therapy in Hormone-Receptor-Positive Breast Cancer: From Tumor Biology to the Rationale for Therapeutic Tunning
Abstract
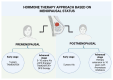
Background and Objectives: The objective of this review is to evaluate the current evidence regarding hormone treatments for both premenopausal and postmenopausal women with early-stage hormone receptor (HR) positive breast cancer. Materials and Methods: An in-depth exploration of the existing literature was conducted, with landmark clinical trials such as TEXT, SOFT, ATLAS, and aTTom serving as primary references. Results: Through an extensive review of the literature, our findings indicate that for premenopausal women with HR-positive, HER2-negative BC with a low risk of recurrence, standard 5-year monotherapy with tamoxifen represents the optimal therapeutic management, given its favorable clinical outcomes and lower associated toxicity. In contrast, for premenopausal women with an intermediate to high risk of recurrence with the same tumor characteristics, the most effective approach stated in the literature is a combination of ovarian suppression therapy (chemical/surgical) and an aromatase inhibitor/selective estrogen receptor modulator (tamoxifen), with a possible extension of the standard therapeutic period. In postmenopausal patients with HR-positive, HER2-negative breast cancer with a low recurrence risk, the first line of treatment is usually a standard 5-year period of treatment with aromatase inhibitors (AIs)(letrozole, anastrozole, or exemestane). On the other hand, in postmenopausal women with an intermediate to high risk, combination therapy might be needed, as well as an extension of the standard therapeutic time. Conclusions: Treatment consensus depends on pre- vs. postmenopausal status and recurrence risk.
Keywords: aromatase inhibitors; breast cancer; hormone therapy; tamoxifen.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Hormonal therapies for early breast cancer: systematic review and economic evaluation.Health Technol Assess. 2007 Jul;11(26):iii-iv, ix-xi, 1-134. doi: 10.3310/hta11260. Health Technol Assess. 2007. PMID: 17610808
-
LHRH agonists for adjuvant therapy of early breast cancer in premenopausal women.Cochrane Database Syst Rev. 2009 Oct 7;2009(4):CD004562. doi: 10.1002/14651858.CD004562.pub4. Cochrane Database Syst Rev. 2009. PMID: 19821328 Free PMC article.
-
Aromatase inhibitors for treatment of advanced breast cancer in postmenopausal women.Cochrane Database Syst Rev. 2007 Jan 24;(1):CD003370. doi: 10.1002/14651858.CD003370.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2009 Oct 07;(4):CD003370. doi: 10.1002/14651858.CD003370.pub3. PMID: 17253488 Updated.
-
Breast Cancer Index in Premenopausal Women With Early-Stage Hormone Receptor-Positive Breast Cancer.JAMA Oncol. 2024 Oct 1;10(10):1379-1389. doi: 10.1001/jamaoncol.2024.3044. JAMA Oncol. 2024. PMID: 39145953 Free PMC article. Clinical Trial.
-
Risk-reducing medications for primary breast cancer: a network meta-analysis.Cochrane Database Syst Rev. 2019 Apr 29;4(4):CD012191. doi: 10.1002/14651858.CD012191.pub2. Cochrane Database Syst Rev. 2019. PMID: 31032883 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

