Diagnostic Approaches for Candida auris: A Comprehensive Review of Screening, Identification, and Susceptibility Testing
- PMID: 40731971
- PMCID: PMC12298640
- DOI: 10.3390/microorganisms13071461
Diagnostic Approaches for Candida auris: A Comprehensive Review of Screening, Identification, and Susceptibility Testing
Abstract
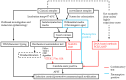
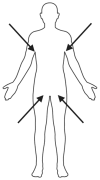
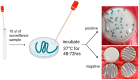
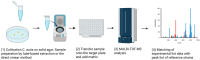
Candida auris (C. auris) is an emerging multidrug-resistant fungal pathogen recognized by the World Health Organization (WHO) as a critical global health threat. Its rapid transmission, high mortality rate, and frequent misidentification in clinical laboratories present significant challenges for diagnosis and infection control. This review provides a comprehensive overview of current and emerging diagnostic methods for C. auris detection, including culture-based techniques, biochemical assays, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS), and molecular diagnostics such as PCR and loop-mediated isothermal amplification (LAMP). We evaluate each method's sensitivity, specificity, turnaround time, and feasibility in clinical and surveillance settings. While culture remains the diagnostic gold standard, it is limited by slow turnaround and phenotypic overlap with related species. Updated biochemical platforms and MALDI-TOF MS with expanded databases have improved identification accuracy. Molecular assays offer rapid, culture-independent detection. Antifungal susceptibility testing (AFST), primarily using broth microdilution, is essential for guiding treatment, although standardized breakpoints remain lacking. This review proposes an integrated diagnostic workflow and discusses key innovations and gaps in current practice. Our findings aim to support clinicians, microbiologists, and public health professionals in improving early detection, containment, and management of C. auris infections.
Keywords: Candida auris; diagnostic methods; matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Rapid detection of rifampin resistance in Mycobacterium tuberculosis using nucleotide MALDI-TOF MS: a comparative study with phenotypic drug susceptibility testing and DNA sequencing.Microbiol Spectr. 2025 Jul;13(7):e0048325. doi: 10.1128/spectrum.00483-25. Epub 2025 May 30. Microbiol Spectr. 2025. PMID: 40445194 Free PMC article.
-
Development of reverse transcription loop-mediated isothermal amplification-based assay for rapid and specific detection of human fungal pathogen, Candida auris.Curr Med Mycol. 2024 Nov 14;10:e2024.345284.1572. doi: 10.22034/cmm.2024.345284.1572. eCollection 2024. Curr Med Mycol. 2024. PMID: 40584605 Free PMC article.
-
Evaluation of the first Candida auris isolates reported from Türkiye in terms of identification by various methods and susceptibility to antifungal drugs.Indian J Med Microbiol. 2024 May-Jun;49:100594. doi: 10.1016/j.ijmmb.2024.100594. Epub 2024 Apr 25. Indian J Med Microbiol. 2024. PMID: 38636843
-
Effectiveness of Practices To Increase Timeliness of Providing Targeted Therapy for Inpatients with Bloodstream Infections: a Laboratory Medicine Best Practices Systematic Review and Meta-analysis.Clin Microbiol Rev. 2016 Jan;29(1):59-103. doi: 10.1128/CMR.00053-14. Clin Microbiol Rev. 2016. PMID: 26598385 Free PMC article.
-
Diagnosing invasive fungal infections in the laboratory today: It's all good news?Rev Iberoam Micol. 2025 Jan-Mar;42(1):1-14. doi: 10.1016/j.riam.2025.01.004. Epub 2025 Mar 19. Rev Iberoam Micol. 2025. PMID: 40268631 Review.
References
Publication types
LinkOut - more resources
Full Text Sources

