Germinated Spores of the Probiotic Bacterium Bacillus coagulans JBI-YZ6.3 Support Dynamic Changes in Intestinal Epithelial Communication and Resilience to Mechanical Wounding
- PMID: 40731976
- PMCID: PMC12298105
- DOI: 10.3390/microorganisms13071466
Germinated Spores of the Probiotic Bacterium Bacillus coagulans JBI-YZ6.3 Support Dynamic Changes in Intestinal Epithelial Communication and Resilience to Mechanical Wounding
Abstract
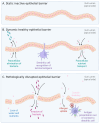
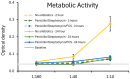
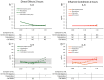
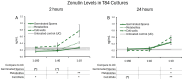
The spore-forming probiotic Bacillus coagulans JBI-YZ6.3 interacts with the gut epithelium via its secreted metabolites as well as its cell walls, engaging pattern-recognition receptors on the epithelium. We evaluated its effects on human T84 gut epithelial cells using in vitro co-cultures, comparing metabolically active germinated spores to the isolated metabolite fraction and cell wall fraction under unstressed versus inflamed conditions. Germinated spores affected epithelial communication via chemokines interleukin-8, interferon gamma-induced protein-10, and macrophage inflammatory protein-1 alpha and beta after 2 and 24 h of co-culture. Non-linear dose responses confirmed that bacterial density affected the epigenetic state of the epithelial cells. In contrast, the cell wall fraction increased cytokine and chemokine levels under both normal and inflamed conditions, demonstrating that the intact bacterium had anti-inflammatory properties, regulating pro-inflammatory signals from its cell walls. During recovery from mechanical wounding, germinated spores accelerated healing, both in the absence and presence of LPS-induced inflammation; both the metabolite and cell wall fractions contributed to this effect. The release of zonulin, a regulator of tight junction integrity, was reduced by germinated spores after 2 h. These findings suggest that B. coagulans JBI-YZ6.3 modulates epithelial chemokine signaling, supports barrier integrity, and enhances epithelial resilience, highlighting its potential as an efficacious multi-faceted probiotic for gut health.
Keywords: IL-6; IL-8; IP-10; MIP-1α; MIP-1β; anti-inflammatory; chemotactic recruitment; wound healing; zonulin.
Conflict of interest statement
The authors declare no conflicts of interest. All authors are affiliated with NIS Labs. Jeneil Biotech Inc. sponsored the research for this work to be performed at NIS Labs. The sponsor had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





Similar articles
-
Differential Immune-Modulating Activities of Cell Walls and Secreted Metabolites from Probiotic Bacillus coagulans JBI-YZ6.3 under Normal versus Inflamed Culture Conditions.Microorganisms. 2023 Oct 15;11(10):2564. doi: 10.3390/microorganisms11102564. Microorganisms. 2023. PMID: 37894222 Free PMC article.
-
In vitro investigation of monoglycerides and zinc glycinate: anti-inflammatory and epithelial barrier function.J Anim Sci. 2023 Jan 3;101:skae372. doi: 10.1093/jas/skae372. J Anim Sci. 2023. PMID: 39657118
-
Exploring the beneficial effects of GHK-Cu on an experimental model of colitis and the underlying mechanisms.Front Pharmacol. 2025 Jul 2;16:1551843. doi: 10.3389/fphar.2025.1551843. eCollection 2025. Front Pharmacol. 2025. PMID: 40672369 Free PMC article.
-
Health professionals' experience of teamwork education in acute hospital settings: a systematic review of qualitative literature.JBI Database System Rev Implement Rep. 2016 Apr;14(4):96-137. doi: 10.11124/JBISRIR-2016-1843. JBI Database System Rev Implement Rep. 2016. PMID: 27532314
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

