Tick-Borne Viruses in a Changing Climate: The Expanding Threat in Africa and Beyond
- PMID: 40732018
- PMCID: PMC12299523
- DOI: 10.3390/microorganisms13071509
Tick-Borne Viruses in a Changing Climate: The Expanding Threat in Africa and Beyond
Abstract
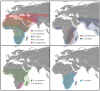
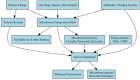
Tick-borne viruses (TBVs), notably Orthonairovirus haemorrhagiae (Crimean-Congo hemorrhagic fever virus, CCHFV), are emerging global health threats intensified by climate change. Rising temperatures and altered precipitation patterns are expanding the habitats of key tick vectors, increasing their survival and reproductive success. The African continent is characterized by many different climatic zones, and climatic shifts have increased or changed CCHFV transmission patterns, becoming greater risk to humans and livestock. Beyond Africa, CCHFV spread in Europe, the Middle East, and Asia and has been facilitated by factors such as livestock movement, deforestation, and migratory birds. Climate-driven shifts in tick seasonality, behavior, and vector competence may further enhance viral transmission. Addressing these challenges requires integrated responses, including enhanced surveillance, predictive modeling, and climate-adaptive vector control strategies. A One Health approach-linking environmental, animal, and human health domains-is essential. Innovative strategies such as anti-tick vaccines and sustainable vector control methods offer promise in reducing the burden of these diseases. Proactive, collaborative efforts at regional and international levels are crucial in tackling this growing public health challenge.
Keywords: Africa; Crimean–Congo hemorrhagic fever virus; climate change; tick-borne viruses.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
The Growing Threat of Tick-Borne Viruses: Global Trends, Clinical Outcomes, and Diagnostic Strategies.Viral Immunol. 2025 May;38(4):125-136. doi: 10.1089/vim.2025.0019. Epub 2025 Apr 24. Viral Immunol. 2025. PMID: 40274388 Review.
-
Zoonotic arbovirus infections in cattle in Mozambique with special reference to Crimean-Congo hemorrhagic fever virus (CCHFV) and rift valley fever virus (RVFV).Virol J. 2025 Jun 6;22(1):185. doi: 10.1186/s12985-025-02804-9. Virol J. 2025. PMID: 40481487 Free PMC article.
-
Tick-Borne Diseases in Sub-Saharan Africa: A Systematic Review of Pathogens, Research Focus, and Implications for Public Health.Pathogens. 2024 Aug 17;13(8):697. doi: 10.3390/pathogens13080697. Pathogens. 2024. PMID: 39204297 Free PMC article.
-
Immunogenicity of NSDV GP38 and the role of furin in GP38 proteolytic processing.J Virol. 2025 Jul 22;99(7):e0053725. doi: 10.1128/jvi.00537-25. Epub 2025 Jun 10. J Virol. 2025. PMID: 40492757 Free PMC article.
-
Climate Change and the Impact on Ocular Infectious Diseases: A Narrative Review.Ophthalmol Ther. 2025 Aug;14(8):1695-1712. doi: 10.1007/s40123-025-01185-0. Epub 2025 Jul 2. Ophthalmol Ther. 2025. PMID: 40601204 Free PMC article. Review.
References
-
- Lizinfeld I., Pshenichnaya N., Parolina L. A comprehensive analysis of climate change impact on crimean-congo hemorrhagic fever spread (Russia, Kazakhstan, Turkey and Iran from 1999 to 2022) Int. J. Infect. Dis. 2025;152:107465. doi: 10.1016/j.ijid.2024.107465. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

