Antiviral Immune Responses Against Murine Cytomegalovirus Induced by an Oral Salmonella-Based Vaccine Expressing Viral M33 Protein
- PMID: 40732019
- PMCID: PMC12300953
- DOI: 10.3390/microorganisms13071510
Antiviral Immune Responses Against Murine Cytomegalovirus Induced by an Oral Salmonella-Based Vaccine Expressing Viral M33 Protein
Abstract
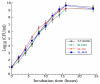
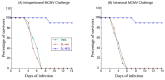
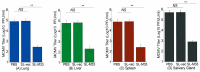
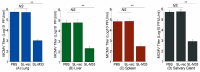
Human cytomegalovirus (CMV) is the leading cause of congenital infections, often leading to mental retardation and neurological disorders. It is a major public health priority to develop a vaccine for preventing and controlling human CMV infection. In this report, we generated an oral Salmonella-based vaccine to express the M33 protein of murine cytomegalovirus (MCMV) and investigated the anti-MCMV immune responses induced in mice immunized with this vaccine. Compared to those administered with phosphate-buffered saline (PBS) or a control vaccine without M33 expression, mice immunized with the vaccine expressing the M33 protein exhibited a remarkable induction of antiviral serum IgG and mucosal IgA humoral responses and a significant elicitation of antiviral T cell responses. Successful inhibition of viral growth in lungs, spleens, livers, and salivary glands was also found in the vaccinated animals compared to the PBS-treated animals or those immunized with the control vaccine without M33 expression. Furthermore, substantial protection against MCMV challenge was observed in mice immunized with the vaccine. Thus, Salmonella-based vaccine expressing MCMV M33 can induce anti-MCMV effective immune responses and protection. Our study implies that attenuated Salmonella expressing human CMV antigens, including its homologue to M33, may represent promising oral anti-CMV vaccine candidates.
Keywords: Salmonella; cytomegalovirus; herpesvirus; immune responses; murine cytomegalovirus; oral vaccine; vaccine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Oral Vaccination with Attenuated Salmonella Expressing Viral M25 Protein Effectively Protects Mice Against Murine Cytomegalovirus Infection.Pathogens. 2025 Mar 25;14(4):314. doi: 10.3390/pathogens14040314. Pathogens. 2025. PMID: 40333046 Free PMC article.
-
Replication-deficient whole-virus vaccines against cytomegalovirus induce protective immunity in a guinea pig congenital infection model.J Virol. 2025 Jul 22;99(7):e0020725. doi: 10.1128/jvi.00207-25. Epub 2025 Jun 11. J Virol. 2025. PMID: 40497723 Free PMC article.
-
Multiantigenic Modified Vaccinia Virus Ankara Vaccine Vectors To Elicit Potent Humoral and Cellular Immune Reponses against Human Cytomegalovirus in Mice.J Virol. 2018 Sep 12;92(19):e01012-18. doi: 10.1128/JVI.01012-18. Print 2018 Oct 1. J Virol. 2018. PMID: 30045984 Free PMC article.
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
COVID-19 Vaccines.2025 Jul 15. Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006–. 2025 Jul 15. Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006–. PMID: 33355732 Free Books & Documents. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

