Genetic Correlates of Synergy Mechanisms of Daptomycin Plus Fosfomycin in Daptomycin-Susceptible and -Resistant Methicillin-Resistant Staphylococcus aureus (MRSA)
- PMID: 40732039
- PMCID: PMC12299106
- DOI: 10.3390/microorganisms13071532
Genetic Correlates of Synergy Mechanisms of Daptomycin Plus Fosfomycin in Daptomycin-Susceptible and -Resistant Methicillin-Resistant Staphylococcus aureus (MRSA)
Abstract
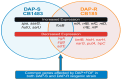
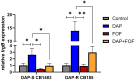
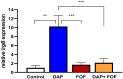
This study elucidates potential genetic determinants and mechanisms involved in the synergistic effects of daptomycin (DAP) + fosfomycin (FOF) combination therapy. Among 33 clinically derived DAP-susceptible (S)/DAP-resistant (R) isogenic strain pairs, mutations in the mprF gene occurred in 30/33 DAP-R strains, including polymorphisms of L826F (33%) or T345A/L/I (15%). Strain variants of DAP-S CB1483 serially passaged in vitro for 10 days in DAP +/- FOF identified a key non-synonymous mutation in mprF (L826F) only in the DAP monotherapy arm. Interestingly, passage in FOF alone or DAP + FOF prevented the emergence of this mprF mutation following 10-day passage. This L826F mprF polymorphism, associated with a "gain-in-function" phenotype, exhibited increased amounts of lysyl-phosphatidylglycerol (L-PG) in the cell membrane (CM). Transcriptomics revealed a relatively modest number (~10) of distinct genes that were significantly up- or downregulated (≥2 log fold) in both the DAP-S and DAP-R strain pairs upon DAP + FOF exposures (vs. DAP or FOF alone). Of note, DAP + FOF decreased expression of lrgAB and sdrE and increased the expression level of fosB. In a rabbit infective endocarditis (IE) model, the DAP-R CB185 strain treated with DAP +/- FOF showed significantly reduced lrgB expression in vegetations compared with DAP treatment alone. Overall, these findings indicate that DAP + FOF therapy impacts MRSA through multiple specific mechanisms, enhancing bacterial clearance.
Keywords: MRSA; combination therapy; daptomycin; fosfomycin; transcriptomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Holland T.L., Cosgrove S.E., Doernberg S.B., Jenkins T.C., Turner N.A., Boucher H.W., Pavlov O., Titov I., Kosulnykov S., Atanasov B., et al. ERADICATE Study Group. Ceftobiprole for treatment of complicated staphylococcus aureus bacteremia. N. Engl. J. Med. 2023;389:1390–1401. doi: 10.1056/NEJMoa2300220. - DOI - PubMed
-
- Fowler V.G., Jr., Boucher H.W., Corey G.R., Abrutyn E., Karchmer A.W., Rupp M.E., Levine D.P., Chambers H.F., Tally F.P., Vigliani G.A., et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 2006;355:653–665. doi: 10.1056/NEJMoa053783. - DOI - PubMed
-
- Rose W.E., Fallon M., Moran J.J., Vanderloo J.P. Vancomycin tolerance in methicillin-resistant Staphylococcus aureus: Influence of vancomycin, daptomycin, and telavancin on differential resistance gene expression. Antimicrob. Agents Chemother. 2012;56:4422–4427. doi: 10.1128/AAC.00676-12. - DOI - PMC - PubMed
-
- Marty F.M., Yeh W.W., Wennersten C.B., Venkataraman L., Albano E., Alyea E.P., Gold H.S., Baden L.R., Pillai S.K. Emergence of a clinical daptomycin-resistant Staphylococcus aureus isolate during treatment of methicillin-resistant Staphylococcus aureus bacteremia and osteomyelitis. J. Clin. Microbiol. 2006;44:595–597. doi: 10.1128/JCM.44.2.595-597.2006. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

