Biosurfactant Produced by Bacillus subtilis UCP 1533 Isolated from the Brazilian Semiarid Region: Characterization and Antimicrobial Potential
- PMID: 40732057
- PMCID: PMC12299173
- DOI: 10.3390/microorganisms13071548
Biosurfactant Produced by Bacillus subtilis UCP 1533 Isolated from the Brazilian Semiarid Region: Characterization and Antimicrobial Potential
Abstract
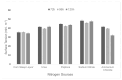
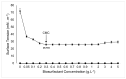
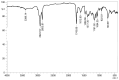
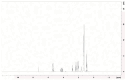
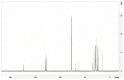
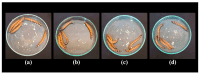
The increasing resistance of pathogenic microorganisms to antimicrobials has driven the search for safe and sustainable alternatives. In this context, microbial biosurfactants have gained prominence due to their antimicrobial activity, low toxicity, and high stability under extreme conditions. This study presents the production and characterization of a biosurfactant with antimicrobial potential, obtained from Bacillus subtilis isolated from soil, for application in the control of resistant strains. Bacterial identification was performed using mass spectrometry (MALDI-TOF), confirming it as Bacillus subtilis. The strain B. subtilis UCP 1533 was cultivated using different carbon sources (glucose, soybean oil, residual frying oil, and molasses) and nitrogen sources (ammonium chloride, sodium nitrate, urea, and peptone), with evaluations at 72, 96, and 120 h. The best condition involved a mineral medium supplemented with 2% soybean oil and 0.12% corn steep liquor, resulting in the production of 16 g·L-1 of biosurfactant, with a critical micelle concentration (CMC) of 0.3 g·L-1 and a reduction in water surface tension to 25 mN·m-1. The biosurfactant showed an emulsification index of 100% for used motor oil and ranged from 50% to 100% for different vegetable oils, maintaining stability across a wide range of pH, salinity, and temperature. FT-IR and NMR analyses confirmed its lipopeptide nature and anionic charge. Toxicity tests with Tenebrio molitor larvae showed 100% survival at all the tested concentrations. In phytotoxicity assays, seed germination rates above 90% were recorded for Solanum lycopersicum and Lactuca sativa. Antimicrobial tests revealed inhibitory activity against resistant strains of Escherichia coli and Pseudomonas aeruginosa, as well as against species of the genus Candida (C. glabrata, C. lipolytica, C. bombicola, and C. guilliermondii), highlighting the biosurfactant as a promising alternative in combating antimicrobial resistance (AMR). These results indicate the potential application of this biosurfactant in the development of antimicrobial agents for pharmaceutical formulations and sustainable strategies for phytopathogen control in agriculture.
Keywords: Bacillus subtilis; antibacterial biosurfactant; antimicrobial resistance; caatinga soil.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Benali T., Lemhadri A., Harboul K., Chtibi H., Khabbach A., Jadouali S.M., Quesada-Romero L., Louahlia S., Hammani K., Ghaleb A., et al. Chemical profiling and biological properties of essential oils of Lavandula stoechas L. collected from three Moroccan sites: In vitro and in silico investigations. Plants. 2023;12:1413. doi: 10.3390/plants12061413. - DOI - PMC - PubMed
-
- Elghoul M., Bouassida M., Ghribi D., Mnif I. A new bacterial-derived biosurfactant for biotechnological applications in the oil industry: Production, optimization, biosurfactant functional and physicochemical characterization. Water Pract. Technol. 2023;18:810–830. doi: 10.2166/wpt.2023.036. - DOI
LinkOut - more resources
Full Text Sources

