Dietary Supplementation with Encapsulated or Non-Encapsulated Sodium Butyrate Enhances Growth, Antioxidant Defense, Immunity, and Gut Health in Largemouth Bass (Micropterus salmoides)
- PMID: 40732103
- PMCID: PMC12300178
- DOI: 10.3390/microorganisms13071594
Dietary Supplementation with Encapsulated or Non-Encapsulated Sodium Butyrate Enhances Growth, Antioxidant Defense, Immunity, and Gut Health in Largemouth Bass (Micropterus salmoides)
Abstract
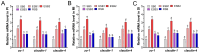
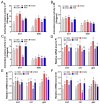
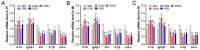
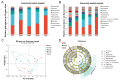
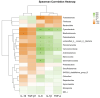
This study aimed to evaluate the effects of dietary supplementation with sodium butyrate (SB) in different forms on the growth performance, antioxidant capacity, immune response, and intestinal health of largemouth bass (Micropterus salmoides). Five diets were formulated: a basal diet (SB0), diets with 1000 (ESB1), 1500 (ESB2), and 2000 mg/kg encapsulated SB (ESB3), and a diet with 2000 mg/kg raw powder sodium butyrate (RSB, non-encapsulated). After 49 days of feeding trials, the ESB2 group exhibited significantly higher weight gain and specific growth rates and a lower feed coefficient than those of the SB0 group (p < 0.05). Compared with the SB0 group, proximal intestinal villus length and width were significantly increased in the ESB1, ESB2, and ESB3 groups (p < 0.05). The expressions of tight junction genes zo-1, claudin-1, and claudin-4 were up-regulated in these SB-supplemented groups and most pronounced in the ESB2 group (p < 0.05). Compared with the SB0 group, antioxidant enzyme activities (catalase and superoxide dismutase) and their gene expressions increased in the ESB1, ESB2, and RSB groups (p < 0.05). Immune-related genes il-10 and tgf-β1 were up-regulated in the ESB1 and ESB2 groups, while their il-8, il-1β, and tnf-α were down-regulated (p < 0.05). The ESB2 group had higher intestinal abundance of Firmicutes and Lactobacillus. In conclusion, dietary supplementation with 1500 mg/kg encapsulated SB (ESB2) improved growth, antioxidant capacity, immunity, and gut health in largemouth bass.
Keywords: Micropterus salmoides; antioxidant enzymes; encapsulation; growth performance; gut microbiota; immunity; sodium butyrate.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Dietary phytosterols increased the rate of weight gain, antioxidant capacity and growth of beneficial strains of bacteria in the gut and suppressed the population of potentially pathogenic bacteria in largemouth bass (Micropterus salmoides).J Anim Sci. 2025 Jan 4;103:skaf011. doi: 10.1093/jas/skaf011. J Anim Sci. 2025. PMID: 39844348
-
Dietary sanguinarine enhances disease resistance to Aeromonas dhakensis in largemouth (Micropterus salmoides).Fish Shellfish Immunol. 2025 Oct;165:110577. doi: 10.1016/j.fsi.2025.110577. Epub 2025 Jul 18. Fish Shellfish Immunol. 2025. PMID: 40684960
-
Multi-omics approach to study the dual effects of novel proteins on the intestinal health of juvenile largemouth bass (Micropterus salmoides) under an alternate feeding strategy.Front Immunol. 2023 Mar 1;14:1110696. doi: 10.3389/fimmu.2023.1110696. eCollection 2023. Front Immunol. 2023. PMID: 36936939 Free PMC article.
-
Effects of replacing zinc oxide with different levels of zinc lactate on growth performance, serum indexes, intestinal health and gut microbiota in weaned piglets.Front Microbiol. 2025 Jul 11;16:1622700. doi: 10.3389/fmicb.2025.1622700. eCollection 2025. Front Microbiol. 2025. PMID: 40718813 Free PMC article. Review.
-
Corticosteroids for the treatment of Duchenne muscular dystrophy.Cochrane Database Syst Rev. 2016 May 5;2016(5):CD003725. doi: 10.1002/14651858.CD003725.pub4. Cochrane Database Syst Rev. 2016. PMID: 27149418 Free PMC article.
References
-
- Jannathulla R., Rajaram V., Kalanjiam R., Ambasankar K., Muralidhar M., Dayal J.S. Fishmeal availability in the scenarios of climate change: Inevitability of fishmeal replacement in aquafeeds and approaches for the utilization of plant protein sources. Aquac. Res. 2019;50:3493–3506. doi: 10.1111/are.14324. - DOI
-
- Krogdahl A., Penn M., Thorsen J., Refstie S., Bakke A.M. Important antinutrients in plant feedstuffs for aquaculture: An update on recent findings regarding responses in salmonids. Aquac. Res. 2010;41:333–344. doi: 10.1111/j.1365-2109.2009.02426.x. - DOI
-
- Hossain M., Pandey A., Satoh S. Effects of organic acids on growth and phosphorus utilization in red sea bream Pagrus major. Fish. Sci. 2007;73:1309–1317. doi: 10.1111/j.1444-2906.2007.01469.x. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

