IL-24 Is a Promising Molecular Adjuvant for Enhancing Protective Immunity Induced by DNA Vaccination Against Toxoplasma gondii
- PMID: 40732170
- PMCID: PMC12299984
- DOI: 10.3390/microorganisms13071661
IL-24 Is a Promising Molecular Adjuvant for Enhancing Protective Immunity Induced by DNA Vaccination Against Toxoplasma gondii
Abstract
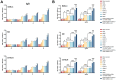
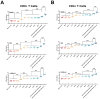
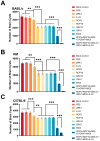
Toxoplasma gondii, a parasitic protozoan, causes zoonotic infections with severe health impacts in humans and warm-blooded animals, underscoring the urgent need for effective vaccines to control these infections. In this study, a DNA vaccine encoding TgROP5, TgROP18, TgGRA7, TgGRA15, and TgMIC6 was formulated using the eukaryotic expression vector pVAX I. IL-24 was delivered as a molecular adjuvant using plasmid pVAX-IL-24. BALB/c, C57BL/6, and Kunming mouse strains received the DNA immunization, after which antibody levels, cytokine production, and lymphocyte surface markers were analyzed to assess immune responses. Additionally, survival rates and brain cyst counts were measured 1 to 2 months post-vaccination in experimental models of toxoplasmosis. As a result, compared to controls, the DNA vaccine cocktail significantly increased serum IgG levels, Th1 cytokine production, and proportions of CD4+/CD8+ T cells, leading to extended survival and reduced brain cyst counts post-challenge with T. gondii ME49. Furthermore, the five-gene DNA vaccine cocktail conferred greater protection compared to single-gene immunizations. Co-administration of IL-24 significantly enhanced the immune efficacy of the multi-gene DNA vaccination. Our findings suggest that IL-24 is an effective molecular adjuvant, enhancing the protective immunity of DNA vaccines against T. gondii, supporting its potential role in vaccine strategies targeting other apicomplexan parasites.
Keywords: T-cell activation; Th1 immunity; protective efficacy; protozoan vaccine; serological markers.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
A novel combined quadrivalent self-amplifying mRNA-LNP vaccine provokes protective immunity against acute and chronic toxoplasmosis in mice.Infect Dis Poverty. 2025 Jun 23;14(1):55. doi: 10.1186/s40249-025-01332-6. Infect Dis Poverty. 2025. PMID: 40551280 Free PMC article.
-
IL-36 Gamma: A Novel Adjuvant Cytokine Enhancing Protective Immunity Induced by DNA Immunization with TGIST and TGNSM Against Toxoplasma gondii Infection in Mice.Microorganisms. 2024 Nov 7;12(11):2258. doi: 10.3390/microorganisms12112258. Microorganisms. 2024. PMID: 39597646 Free PMC article.
-
Immunogenicity and Protective Efficacy of a Recombinant Toxoplasma gondii GRA12 Vaccine in Domestic Cats.Vaccines (Basel). 2025 Aug 11;13(8):851. doi: 10.3390/vaccines13080851. Vaccines (Basel). 2025. PMID: 40872935 Free PMC article.
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
Investigating factors affecting the effectiveness of Gardasil 4, Cervarix, and Gardasil 9 vaccines considering the WHO regions in females: A systematic review.Cancer Epidemiol. 2025 Apr;95:102759. doi: 10.1016/j.canep.2025.102759. Epub 2025 Feb 5. Cancer Epidemiol. 2025. PMID: 39914284
References
-
- Bisetegn H., Debash H., Ebrahim H., Mahmood N., Gedefie A., Tilahun M., Alemayehu E., Mohammed O., Feleke D.G. Global seroprevalence of Toxoplasma gondii infection among patients with mental and neurological disorders: A systematic review and meta-analysis. Health Sci. Rep. 2023;6:e1319. doi: 10.1002/hsr2.1319. - DOI - PMC - PubMed
-
- Du R., He J., Meng J., Zhang D., Li D., Wang H., Fan A., Xu G., Ma S., Zuo Z., et al. Vaccination with a DNA vaccine cocktail encoding TgROP2, TgROP5, TgROP9, TgROP16, TgROP17, and TgROP18 confers limited protection against Toxoplasma gondii in BALB/c mice. Parasitol. Res. 2024;123:420. doi: 10.1007/s00436-024-08435-3. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

