Virulence, Antibiotic Resistance and Cytotoxic Effects of Lactococcus lactis Isolated from Chinese Cows with Clinical Mastitis on MAC-T Cells
- PMID: 40732181
- PMCID: PMC12298479
- DOI: 10.3390/microorganisms13071674
Virulence, Antibiotic Resistance and Cytotoxic Effects of Lactococcus lactis Isolated from Chinese Cows with Clinical Mastitis on MAC-T Cells
Abstract
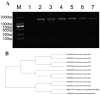
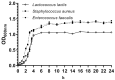
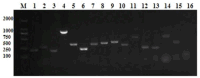
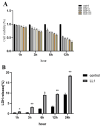
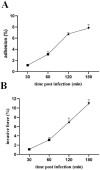
Lactococcus lactis (L. lactis) is a pathogenic Gram-positive, catalase-negative coccobacillus (GPCN) associated with bovine mastitis. In this study, nine strains of L. lactis were successfully isolated and characterized from 457 milk samples from cows with clinical mastitis in China. All isolates exhibited a high degree of susceptibility to marbofloxacin and vancomycin. A series of molecular and cell biological techniques were used to explore the biological characteristics and pathogenicity of these isolates. The virulence gene profiles of the isolates were analyzed using whole genome resequencing combined with polymerase chain reaction (PCR) to elucidate the differences in virulence gene expression between isolates. To provide a more visual demonstration of the pathogenic effect of L. lactis on bovine mammary epithelial cells, an in vitro infection model was established using MAC-T cells. The results showed that L. lactis rapidly adhered to the surface of bovine mammary epithelial cells and significantly induced the release of lactate dehydrogenase, suggesting that the cell membranes might be damaged. Ultrastructural observations showed that L. lactis not only adhered to MAC-T cells, but also invaded the cells through a perforation mechanism, leading to a cascade of organelle damage, including mitochondrial swelling and ribosome detachment from the endoplasmic reticulum. The objective of this study was to provide strong evidence for the cytotoxic effects of L. lactis on bovine mammary epithelial cells. Based on this research, a prevention and treatment strategy for L. lactis as well as major pathogenic mastitis bacteria should be established, and there is a need for continuous monitoring.
Keywords: Lactococcus lactis; antimicrobial resistance; bovine clinical mastitis; pathogenicity; virulence gene.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Akkermansia muciniphila-Derived Outer Membrane Vesicles as a Novel Therapeutic Approach for Mastitis: Insights From In Vitro and Vivo Studies.FASEB J. 2025 Jul 15;39(13):e70770. doi: 10.1096/fj.202500877RR. FASEB J. 2025. PMID: 40586785 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
References
-
- Kim D., Beck B.R., Heo S.B., Kim J., Kim H.D., Lee S.M., Kim Y., Oh S.Y., Lee K., Do H., et al. Lactococcus lactis Bfe920 Activates the Innate Immune System of Olive Flounder (Paralichthys olivaceus), Resulting in Protection against Streptococcus iniae Infection and Enhancing Feed Efficiency and Weight Gain in Large-Scale Field Studies. Fish Shellfish. Immunol. 2013;35:1585–1590. doi: 10.1016/j.fsi.2013.09.008. - DOI - PubMed
-
- Devriese L.A., Hommez J., Laevens H., Pot B., Vandamme P., Haesebrouck F. Identification of Aesculin-Hydrolyzing Streptococci, Lactococci, Aerococci and Enterococci from Subclinical Intramammary Infections in Dairy Cows. Vet. Microbiol. 1999;70:87–94. doi: 10.1016/S0378-1135(99)00124-8. - DOI - PubMed
-
- Werner B., Moroni P., Gioia G., Lavín-Alconero L., Yousaf A., Charter M.E., Carter B.M., Bennett J., Nydam D.V., Welcome F., et al. Short Communication: Genotypic and Phenotypic Identification of Environmental Streptococci and Association of Lactococcus lactis ssp. Lactis with Intramammary Infections among Different Dairy Farms. J. Dairy Sci. 2014;97:6964–6969. doi: 10.3168/jds.2014-8314. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

