Impact of Live Ligilactobacillus salivarius CCFM1332 and Its Postbiotics on Porphyromonas gingivalis Colonization, Alveolar Bone Resorption and Inflammation in a Rat Model of Periodontitis
- PMID: 40732209
- PMCID: PMC12300987
- DOI: 10.3390/microorganisms13071701
Impact of Live Ligilactobacillus salivarius CCFM1332 and Its Postbiotics on Porphyromonas gingivalis Colonization, Alveolar Bone Resorption and Inflammation in a Rat Model of Periodontitis
Abstract
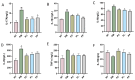
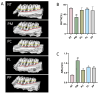
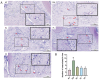
Periodontitis is a chronic inflammatory disease caused by periodontopathic bacteria such as Porphyromonas gingivalis (P. gingivalis), which leads to alveolar bone destruction and systemic inflammation. Emerging evidence suggests that probiotics may mitigate periodontal pathology. To systematically evaluate the alleviative effects and mechanisms of different forms of probiotics, including live bacteria and postbiotics, on periodontitis, we first screened and identified Ligilactobacillus salivarius CCFM1332 (L. salivarius CCFM1332) through in vitro antibacterial and anti-biofilm activity assays. Subsequently, we investigated its therapeutic potential in a rat model of experimental periodontitis. The results demonstrated that both live L. salivarius CCFM1332 (PL) and its postbiotics (PP) significantly reduced the gingival index (GI) and probing depth (PD) in rats, while suppressing oral colonization of P. gingivalis. Serum pro-inflammatory cytokine levels were differentially modulated: the PL group exhibited reductions in interleukin-17A (IL-17A), interleukin-6 (IL-6), and interleukin-1β (IL-1β) by 39.31% (p < 0.01), 17.26% (p < 0.05), and 14.74% (p < 0.05), respectively, whereas the PP group showed decreases of 34.79% (p < 0.05), 29.85% (p < 0.01), and 19.74% (p < 0.05). Micro-computed tomography (Micro-CT) analysis demonstrated that compared to the periodontitis model group (PM), the PL group significantly reduced alveolar bone loss (ABL) by 30.1% (p < 0.05) and increased bone volume fraction (BV/TV) by 49.5% (p < 0.01). In contrast, while the PP group similarly decreased ABL by 32.7% (p < 0.05), it resulted in a 40.4% improvement in BV/TV (p > 0.05). Histological assessments via hematoxylin and eosin (H&E) and tartrate-resistant acid phosphatase (TRAP) staining confirmed that both the PL group and the PP group alleviated structural damage to alveolar bone-supporting tissues and reduced osteoclast-positive cell counts. This study suggests that live L. salivarius CCFM1332 and its postbiotics reduce alveolar bone resorption and attachment loss in rats through antibacterial and anti-inflammatory pathways, thereby alleviating periodontal inflammation in rats.
Keywords: Ligilactobacillus salivarius; alveolar bone resorption; inflammation; periodontitis; postbiotics.
Conflict of interest statement
Authors Qing Hong, Shumao Cui and Zhenmin Liu were employed by the company Bright Dairy & Food Co., Ltd., The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
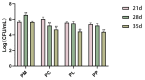

Similar articles
-
Bmi-1 alleviates alveolar bone resorption through the regulation of autophagy.J Periodontol. 2025 Jun;96(6):694-707. doi: 10.1002/JPER.23-0796. Epub 2024 Sep 23. J Periodontol. 2025. PMID: 39311723
-
Immunohistochemical Analysis to Evaluate the Efficacy of Tetracycline-Loaded Nano-Chitosan in Treating Periodontitis Induced by Porphyromonas gingivalis in Albino Rats.Int J Dent. 2025 Jul 14;2025:1959086. doi: 10.1155/ijod/1959086. eCollection 2025. Int J Dent. 2025. PMID: 40692535 Free PMC article.
-
Long noncoding RNA MIR100HG regulates inflammatory response by interacting with RNA-binding protein Quaking in periodontitis.J Periodontol. 2025 Aug 17. doi: 10.1002/jper.11394. Online ahead of print. J Periodontol. 2025. PMID: 40824186
-
Adjunctive antimicrobial photodynamic therapy for treating periodontal and peri-implant diseases.Cochrane Database Syst Rev. 2024 Jul 12;7(7):CD011778. doi: 10.1002/14651858.CD011778.pub2. Cochrane Database Syst Rev. 2024. PMID: 38994711 Free PMC article.
-
Chlorhexidine mouthrinse as an adjunctive treatment for gingival health.Cochrane Database Syst Rev. 2017 Mar 31;3(3):CD008676. doi: 10.1002/14651858.CD008676.pub2. Cochrane Database Syst Rev. 2017. PMID: 28362061 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

