Garlic Peel-Derived Phytochemicals Using GC-MS: Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Effects in Ulcerative Colitis Rat Model
- PMID: 40732258
- PMCID: PMC12298587
- DOI: 10.3390/ph18070969
Garlic Peel-Derived Phytochemicals Using GC-MS: Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Effects in Ulcerative Colitis Rat Model
Abstract
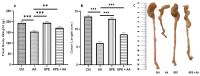
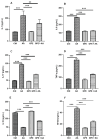
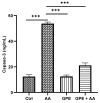
Background/Objectives: Ulcerative colitis (UC) is a chronic, relapsing inflammatory bowel disease (IBD) that poses a significant gastroenterological challenge. Methods: This study investigates the protective effects of garlic peel extract (GPE) in a rat model of acetic acid (AA)-induced colitis. Rats received oral GPE (100 mg/kg) for 14 days prior to AA administration, and this continued for 14 days post-induction. Results: GC-MS analysis of GPE identified several key phytochemicals, primarily methyl esters of fatty acids (62.47%), fatty acids (10.36%), fatty acid derivatives (6.75%), and vitamins (4.86%) as the major constituents. Other notable compounds included steroids, natural alcohols, organosulfur compounds, fatty aldehydes, carotenoids, sugars, and glucosinolates. GPE treatment significantly improved body weight and colon length. Biochemical analysis showed that GPE downregulated the levels of the pro-inflammatory cytokines interleukin-1 (IL-1), IL-6, IL-17, tumor necrosis factor-alpha (TNF-α), and nuclear factor-kappa B (NF-κB), compared to the colitis (AA) group. Additionally, GPE reduced the oxidative stress (OS) biomarkers, including myeloperoxidase (MPO) and malondialdehyde (MDA), as well as caspase-3, a marker for apoptosis. Furthermore, GPE treatment resulted in enhanced activities of the enzymatic antioxidants catalase (CAT) and superoxide dismutase (SOD), along with increased levels of the anti-inflammatory cytokine IL-10. These findings were supported by histological evidence. Conclusions: Collectively, GPE holds promise as a therapeutic strategy for UC, owing to its natural bioactive compounds and their potential synergistic anti-inflammatory, antioxidant, and anti-apoptotic effects.
Keywords: GC-MS; anti-inflammatory; antioxidants; cytokines; fatty acid esters; garlic peel extract; natural therapy; ulcerative colitis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Feng J., Liu Y., Zhang C., Ji M., Li C. Phytic acid regulates proliferation of colorectal cancer cells by downregulating NF-kB and β-catenin signalling. Eur. J. Inflamm. 2023;21:1–14. doi: 10.1177/1721727X231182622. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

