Charged Thienobenzo-1,2,3-Triazoles as Especially Potent Non-Selective Cholinesterase Inhibitors: Design, Anti-Inflammatory Activity, and Computational Study
- PMID: 40732321
- PMCID: PMC12299554
- DOI: 10.3390/ph18071032
Charged Thienobenzo-1,2,3-Triazoles as Especially Potent Non-Selective Cholinesterase Inhibitors: Design, Anti-Inflammatory Activity, and Computational Study
Abstract
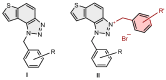
Background/Objectives: This research reports the synthesis and evaluation of novel charged thienobenzo-triazoles as non-selective cholinesterase inhibitors (AChEs and BChEs), their anti-inflammatory properties, and a computational study. Methods: Fifteen derivatives were created through photochemical cyclization and quaternization of the triazole core. The compounds were tested for AChE and BChE inhibition. They showed greater potency and selectivity toward BChE. Results: The most potent compound, derivative 14, inhibited BChE with an IC50 of 98 nM, while derivative 9 also displayed significant anti-inflammatory activity by inhibiting LPS-induced TNF-α production (IC50 = 0.66 µM). Molecular docking revealed that triazolinium salts form key π-π and electrostatic interactions within enzyme active sites. In silico predictions indicated favorable ADME-Tox properties for compounds 9 and 11, including low mutagenicity and moderate CNS permeability. Conclusions: These findings highlight the potential of new charged triazolinium salts as peripherally selective cholinesterase inhibitors with additional anti-inflammatory potential.
Keywords: ADME-Tox prediction; BChE inhibitors; anti-inflammatory activity; cholinesterase; molecular docking; thienobenzo-1,2,3-triazoles.
Conflict of interest statement
The authors Paula Pongrac, Dora Štefok, and Martina Bosnar were employed by the company Selvita Ltd. Zlata Lasić is employed by the Pliva Hrvatska d.o.o. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
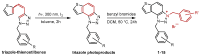





Similar articles
-
Photochemically Assisted Synthesis of Thienobenzotriazole-Based Dual Cholinesterase Inhibitors.Molecules. 2025 Aug 20;30(16):3439. doi: 10.3390/molecules30163439. Molecules. 2025. PMID: 40871591 Free PMC article.
-
Design, synthesis, and biological evaluation of caffeic acid-based novel multifunctional molecules for the management of Alzheimer's disease.Eur J Med Chem. 2025 Oct 15;296:117831. doi: 10.1016/j.ejmech.2025.117831. Epub 2025 Jun 10. Eur J Med Chem. 2025. PMID: 40578253
-
Novel pyrazole carboxylate derivatives as lonazolac bioisosteres with selective COX-2 inhibition: design, synthesis and anti-inflammatory activity.Mol Divers. 2025 May 22. doi: 10.1007/s11030-025-11220-8. Online ahead of print. Mol Divers. 2025. PMID: 40405028
-
Topical anti-inflammatory treatments for eczema: network meta-analysis.Cochrane Database Syst Rev. 2024 Aug 6;8(8):CD015064. doi: 10.1002/14651858.CD015064.pub2. Cochrane Database Syst Rev. 2024. PMID: 39105474 Free PMC article.
-
How lived experiences of illness trajectories, burdens of treatment, and social inequalities shape service user and caregiver participation in health and social care: a theory-informed qualitative evidence synthesis.Health Soc Care Deliv Res. 2025 Jun;13(24):1-120. doi: 10.3310/HGTQ8159. Health Soc Care Deliv Res. 2025. PMID: 40548558
Cited by
-
Photochemically Assisted Synthesis of Thienobenzotriazole-Based Dual Cholinesterase Inhibitors.Molecules. 2025 Aug 20;30(16):3439. doi: 10.3390/molecules30163439. Molecules. 2025. PMID: 40871591 Free PMC article.
References
-
- Talesa V.N. Acetylcholinesterase in Alzheimer’s disease. Curr. Drug Targets. 2001;2:363–373. doi: 10.1016/S0047-6374(01)00309-8. - DOI
-
- Greig N.H., Utsuki T., Yu Q.S., Holloway H.W. A new therapeutic target in Alzheimer’s disease treatment: Selective butyrylcholinesterase inhibition. Curr. Med. Chem. 2005;12:237–243.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

