Combined Antiviral and Cytoprotective Action of Rosmarinic Acid Against EV-A71 Infection: A Potential Therapeutic Strategy
- PMID: 40732670
- PMCID: PMC12299770
- DOI: 10.3390/pathogens14070622
Combined Antiviral and Cytoprotective Action of Rosmarinic Acid Against EV-A71 Infection: A Potential Therapeutic Strategy
Abstract
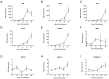
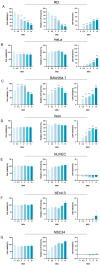
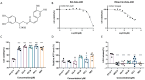
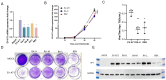
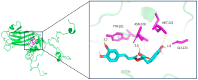
Enterovirus A71 (EV-A71), a major etiological agent of hand-foot-mouth disease, can cause severe neurological complications. However, the mechanisms underlying EV-A71-induced cell damage and potential therapeutic strategies remain inadequately understood. Here, we investigated EV-A71 replication dynamics and associated cytopathic effects in nine distinct cell lines, including epithelial, neuronal, immune, and other cell types. Cell viability, membrane integrity, and energy metabolism were assessed using Cell Counting Kit-8 (CCK-8), lactate dehydrogenase (LDH), and adenosine triphosphate (ATP) assays. The antiviral activity of rosmarinic acid (RA), a natural polyphenol, was evaluated by plaque reduction, qPCR, and Western blot. EV-A71 exhibited cell-type-specific replication and cytotoxicity patterns. RA significantly preserved cell viability, reduced LDH release, maintained ATP levels, and suppressed IL-6 expression. Mechanistically, RA inhibited viral replication by downregulating VP1 expression and viral RNA levels. Molecular docking indicated strong binding of RA to the hydrophobic pocket of VP1, potentially disrupting virus-host interactions. Collectively, these findings highlight RA's combined antiviral and cytoprotective potential, supporting its candidacy as a therapeutic agent against EV-A71 infection.
Keywords: Enterovirus A71; cell damage; inflammatory response; rosmarinic acid; viral replication.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
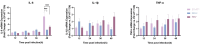
Similar articles
-
Rosmarinic acid exhibits broad anti-enterovirus A71 activity by inhibiting the interaction between the five-fold axis of capsid VP1 and cognate sulfated receptors.Emerg Microbes Infect. 2020 Dec;9(1):1194-1205. doi: 10.1080/22221751.2020.1767512. Emerg Microbes Infect. 2020. PMID: 32397909 Free PMC article.
-
Plasminogen deficiency reduces disease severity and immune responses in enterovirus A71-infected mice.Microbiol Spectr. 2025 Jul;13(7):e0331124. doi: 10.1128/spectrum.03311-24. Epub 2025 May 16. Microbiol Spectr. 2025. PMID: 40377310 Free PMC article.
-
Severe enterovirus A71 infection is associated with dysfunction of T cell immune response and alleviated by Astragaloside A.Virol Sin. 2025 Jun;40(3):451-461. doi: 10.1016/j.virs.2025.05.010. Epub 2025 May 29. Virol Sin. 2025. PMID: 40449890 Free PMC article.
-
Insight into the Life Cycle of Enterovirus-A71.Viruses. 2025 Jan 27;17(2):181. doi: 10.3390/v17020181. Viruses. 2025. PMID: 40006936 Free PMC article. Review.
-
Seroprevalence of coxsackievirus A6 and enterovirus A71 infection in humans: a systematic review and meta-analysis.Arch Virol. 2023 Jan 7;168(2):37. doi: 10.1007/s00705-022-05642-0. Arch Virol. 2023. PMID: 36609748 Free PMC article.
References
-
- Hu Y., Zeng G., Chu K., Zhang J., Han W., Zhang Y., Li J., Zhu F. Five-year immunity persistence following immunization with inactivated enterovirus 71 type (EV71) vaccine in healthy children: A further observation. Hum. Vaccines Immunother. 2018;14:1517–1523. doi: 10.1080/21645515.2018.1442997. - DOI - PMC - PubMed
-
- Cursino C.N., Monteiro P.G.O., Duarte G.D.S., Vieira T.B.Q., Crisante V.C., Giordani F., Xavier A.R., de Almeida R., Calil-Elias S. Predictors of adverse drug reactions associated with ribavirin in direct-acting antiviral therapies for chronic hepatitis C. Pharmacoepidemiol. Drug Saf. 2019;28:1601–1608. doi: 10.1002/pds.4904. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

