The Effect of the Fiber Diameter, Epoxy-to-Amine Ratio, and Degree of PVA Saponification on CO2 Adsorption Properties of Amine-Epoxy/PVA Nanofibers
- PMID: 40732852
- PMCID: PMC12300401
- DOI: 10.3390/polym17141973
The Effect of the Fiber Diameter, Epoxy-to-Amine Ratio, and Degree of PVA Saponification on CO2 Adsorption Properties of Amine-Epoxy/PVA Nanofibers
Abstract
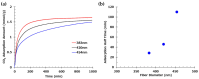
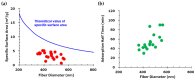
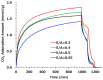
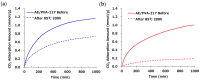
Achieving carbon neutrality requires not only reducing CO2 emissions but also capturing atmospheric CO2. Direct air capture (DAC) using amine-based adsorbents has emerged as a promising approach. In this study, we developed amine-epoxy/poly(vinyl alcohol) (AE/PVA) nanofibers via electrospinning and in situ thermal polymerization. PVA was incorporated to enhance spinnability, and B-staging of AE enabled fiber formation without inline heating. We systematically investigated the effects of electrospinning parameters, epoxy-to-amine ratios (E/A), and the degree of PVA saponification on CO2 adsorption performance. Thinner fibers, obtained by adjusting spinning conditions, exhibited faster adsorption kinetics due to increased surface area. Varying the E/A revealed a trade-off between adsorption capacity and low-temperature desorption efficiency, with secondary amines offering a balanced performance. Additionally, highly saponified PVA improved thermal durability by minimizing side reactions with amines. These findings highlight the importance of optimizing fiber morphology, chemical composition, and polymer properties to enhance the performance and stability of AE/PVA nanofibers for DAC applications.
Keywords: amine-epoxy nanofibers; carbon capture; electrospinning; nanofiber; poly(vinyl alcohol).
Conflict of interest statement
Author Chisato Okada was employed by the company Nitto Denko Corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


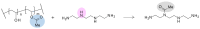
References
-
- Stocker T.F., Qin D., Plattner G.-K., Tignor M., Allen S.K., Boschung J., Nauels A., Xia Y., Bex V., Midgley P.M., editors. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; Cambridge, UK: New York, NY, USA: 2013. 1535p
-
- Lenssen N.J.L., Schmidt G.A., Hansen J.E., Menne M.J., Persin A., Ruedy R., Zyss D. Improvements in the GISTEMP Uncertainty Model. J. Geophys. Res. Atmos. 2019;124:6307–6326. doi: 10.1029/2018JD029522. - DOI
-
- Etheridge D.M., Steele L.P., Langenfelds R.L., Francey R.J., Barnola J.M., Morgan V.I. Historical CO2 Records from the Law Dome DE08, DE08-2, and DSS Ice Cores. J. Geophys. Res. Atmos. 1996;101:4115–4128. doi: 10.1029/95JD03410. - DOI
-
- Shi X., Xiao H., Lackner K.S., Chen B. Tying Amines Down for Stable CO2 Capture. Science. 2020;368:928–932. doi: 10.1126/science.abc9135. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

