Intermittent Fasting as a Neuroprotective Strategy: Gut-Brain Axis Modulation and Metabolic Reprogramming in Neurodegenerative Disorders
- PMID: 40732891
- PMCID: PMC12298811
- DOI: 10.3390/nu17142266
Intermittent Fasting as a Neuroprotective Strategy: Gut-Brain Axis Modulation and Metabolic Reprogramming in Neurodegenerative Disorders
Abstract
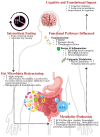
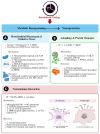
Intermittent fasting (IF) is emerging as a heterogeneous neurometabolic intervention with the possibility of changing the course of neurodegenerative diseases. Through the modulation of the gut-brain axis (GBA), cellular bioenergetics (or metabolic) reprogramming, and involvement in preserved stress adaptation pathways, IF influences a range of physiological mechanisms, including mitobiogenesis, autophagy, circadian rhythm alignment, and neuroinflammation. This review critically synthesises current preclinical and early clinical evidence illustrating IF's capability to supplement synaptic plasticity and integrity, reduce toxic proteins (proteotoxic) burden, and rehabilitate glial and immune homeostasis across models of Alzheimer's disease, Parkinson's disease, Huntington's disease, and amyotrophic lateral sclerosis. The key players behind these effects are bioactive metabolites such as short-chain fatty acids (SCFA) and β-hydroxybutyrate (BHB), and molecular mediators such as brain-derived neurotrophic factor (BDNF). We feature the therapeutic pertinence of IF-induced changes in gut microbiota composition, immune response, and mitochondrial dynamics, and we discuss emerging approaches for merging IF into precision medicine frameworks. Crucial challenges include individual variability, protocol optimisation, safety in cognitively vulnerable populations, and the need for biomarker-guided, ethically grounded clinical trials. Finally, we propose IF as a scalable and flexible intervention that, when personalised and integrated with other modalities, may reframe neurodegeneration from a model of irreversible decline to one of modifiable resilience.
Keywords: SCFAs; autophagy; gut–brain axis; intermittent fasting; metabolic reprogramming; neurodegenerative diseases.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Kormas P., Moutzouri A. Current Psychological Approaches in Neurodegenerative Diseases. In: Vlamos P., Kotsireas I.S., Tarnanas I., editors. Handbook of Computational Neurodegeneration. Springer; Cham, Switzerland: 2022. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

