The Hypoglycaemic Effects of the New Zealand Pine Bark Extract on Sucrose Uptake and Glycaemic Responses in Healthy Adults-A Single-Blind, Randomised, Placebo-Controlled, Crossover Trial
- PMID: 40732901
- PMCID: PMC12298276
- DOI: 10.3390/nu17142277
The Hypoglycaemic Effects of the New Zealand Pine Bark Extract on Sucrose Uptake and Glycaemic Responses in Healthy Adults-A Single-Blind, Randomised, Placebo-Controlled, Crossover Trial
Abstract
Background: The New Zealand pine bark has been demonstrated in vitro to inhibit digestive enzymes involved in carbohydrate digestion (alpha-amylase, alpha-glucosidase, and dipeptidyl-peptidase 4 (DPP-4)).
Objective: This study aims to investigate the inhibitory effects of the New Zealand pine bark on sucrose uptake and glycaemic responses in humans.
Methods: A single-blind, randomised, placebo-controlled, crossover trial was carried out involving healthy adults (n = 40 (M: 12, F: 28), 30.1 ± 1.3 years, BMI 23.4 ± 0.5 kg/m2, HbA1c 32.5 ± 0.6 mmol/mol, FBG 4.7 ± 0.1 mmol/L). A control (75 g of sucrose powder only), and two doses of the pine bark extract (50 and 400 mg) were provided on separate occasions, with 75 g of sucrose mixed in 250 mL of water. Blood samples were collected at -10, 0, 15, 30, 45, 60, 90, and 120 min via a finger prick test. A linear mixed model for repeated measures (SPSS v30, IBM) was applied, and data presented as model-adjusted mean ± SEM.
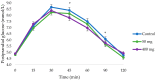
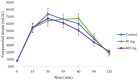
Results: Compared to control (247.5 ± 14.0 mmol/L⋅min), the iAUCglucose was significantly reduced with the 400 mg dose (211.8 ± 13.9 mmol/L⋅min, 14.4% reduction, and p = 0.037), but not with 50 mg dose (220.8 ± 14.2 mmol/L⋅min, 10.8% reduction, and p = 0.184). Compared to control (9.1 ± 0.2 mmol/L), glucose peak value was significantly reduced with the 50 mg dose (8.6 ± 0.2 mmol/L, 5.5% reduction, and p = 0.016) but not with the 400 mg dose (8.7 ± 0.2 mmol/L, 4.4% reduction, and p = 0.093). There were no statistically significant changes in postprandial insulin levels with the pine bark extract compared to control.
Conclusions: The New Zealand pine bark extract attenuated sucrose uptake with improved glycaemic responses, and may therefore be useful as a hypoglycaemic adjunct to the diet.
Keywords: bioactives; glucose intolerance; hyperglycaemia; plant extract; postprandial glucose; postprandial insulin; proanthocyanin; sucrose inhibition; type 2 diabetes.
Conflict of interest statement
The authors declare no conflicts of interest. The authors declare that this study received funding from ENZO Nutraceuticals Limited, Paeroa, New Zealand. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- International Diabetes Federation . IDF Diabetes Atlas. International Diabetes Federation; Brussels, Belgium: 2025.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

