Gut Microbiota Shifts After a Weight Loss Program in Adults with Obesity: The WLM3P Study
- PMID: 40732984
- PMCID: PMC12299616
- DOI: 10.3390/nu17142360
Gut Microbiota Shifts After a Weight Loss Program in Adults with Obesity: The WLM3P Study
Abstract
Background: The gut microbiota is increasingly recognized as a key modulator in obesity management, influencing host energy balance, lipid metabolism, and inflammatory pathways. With obesity prevalence continuing to rise globally, dietary interventions that promote beneficial microbial shifts are essential for enhancing weight loss outcomes and long-term health.
Objective: This study investigated the effects of the multicomponent Weight Loss Maintenance 3 Phases Program (WLM3P), which integrates caloric restriction, a high-protein low-carbohydrate diet, time-restricted eating (10h TRE), dietary supplementation (prebiotics and phytochemicals), and digital app-based support on gut microbiota composition compared to a standard low-carbohydrate diet (LCD) in adults with obesity. The analysis focused exclusively on the 6-month weight loss period corresponding to Phases 1 and 2 of the WLM3P intervention.
Methods: In this sub-analysis of a randomized controlled trial (ClinicalTrials.gov Identifier: NCT04192357), 58 adults with obesity (BMI 30.0-39.9 kg/m2) were randomized to the WLM3P (n = 29) or LCD (n = 29) groups. Stool samples were collected at baseline and 6 months for 16S rRNA sequencing. Alpha and beta diversity were assessed, and genus-level differential abundance was determined using EdgeR and LEfSe. Associations between microbial taxa and clinical outcomes were evaluated using regression models.
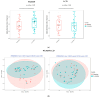
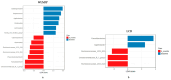
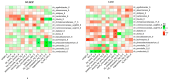
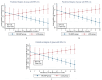
Results: After 6-month, the WLM3P group showed a significant increase in alpha diversity (p = 0.03) and a significant change in beta diversity (p < 0.01), while no significant changes were observed in the LCD group. Differential abundance analysis revealed specific microbial signatures in WLM3P participants, including increased levels of Faecalibacterium. Notably, higher Faecalibacterium abundance was associated with greater reductions in fat mass (kg, %) and visceral adiposity (cm2) in the WLM3P group compared to LCD (p < 0.01).
Conclusions: These findings suggest a potential microbiota-mediated mechanism in weight loss, where Faecalibacterium may enhance fat reduction effectiveness in the context of the WLM3P intervention.
Keywords: Faecalibacterium; dietary interventions; fat mass loss; gut microbiota; visceral fat loss; weight loss.
Conflict of interest statement
Vanessa Pereira work at Farmodiética S.A., one of the study sponsors. The other researchers do not have any relationship with this sponsor.
Figures
References
-
- Ng M., Gakidou E., Lo J., Abate Y.H., Abbafati C., Abbas N., Abbasian M., Abd ElHafeez S., Abdel-Rahman W.M., Abd-Elsalam S., et al. Global, Regional, and National Prevalence of Adult Overweight and Obesity, 1990–2021, with Forecasts to 2050: A Forecasting Study for the Global Burden of Disease Study 2021. Lancet. 2025;405:813–838. doi: 10.1016/S0140-6736(25)00355-1. - DOI - PMC - PubMed
-
- Jensen M.D., Ryan D.H., Apovian C.M., Ard J.D., Comuzzie A.G., Donato K.A., Hu F.B., Hubbard V.S., Jakicic J.M., Kushner R.F., et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. Circulation. 2014;129:S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee. - DOI - PMC - PubMed
-
- Zsálig D., Berta A., Tóth V., Szabó Z., Simon K., Figler M., Pusztafalvi H., Polyák É. A Review of the Relationship between Gut Microbiome and Obesity. Appl. Sci. 2023;13:610. doi: 10.3390/app13010610. - DOI
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

