PET and SPECT Tracer Development via Copper-Mediated Radiohalogenation of Divergent and Stable Aryl-Boronic Esters
- PMID: 40733046
- PMCID: PMC12298831
- DOI: 10.3390/pharmaceutics17070837
PET and SPECT Tracer Development via Copper-Mediated Radiohalogenation of Divergent and Stable Aryl-Boronic Esters
Abstract
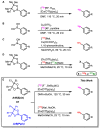
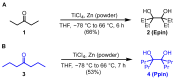
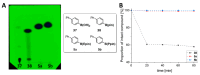
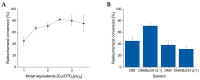
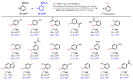
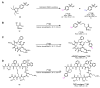
Background/Objectives: Positron emission tomography (PET) and single-photon emission computed tomography (SPECT) are highly sensitive clinical imaging modalities, frequently employed in conjunction with magnetic resonance imaging (MRI) or computed tomography (CT) for diagnosing a wide range of disorders. Efficient and robust radiolabeling methods are needed to accommodate the increasing demand for PET and SPECT tracer development. Copper-mediated radiohalogenation (CMRH) reactions enable rapid late-stage preparation of radiolabeled arenes, yet synthetic challenges and radiolabeling precursors' instability can limit the applications of CMRH approaches. Methods: A series of aryl-boronic acids were converted into their corresponding aryl-boronic acid 1,1,2,2-tetraethylethylene glycol esters [ArB(Epin)s] and aryl-boronic acid 1,1,2,2-tetrapropylethylene glycol esters [ArB(Ppin)s] as stable and versatile precursor building blocks for radiolabeling via CMRH. General protocols for the preparation of 18F-labeled and 123I-labeled arenes utilizing CMRH of these substrates were developed and applied. The radiochemical conversions (RCC) were determined by radio-(U)HPLC. Results: Both ArB(Epin)s and ArB(Ppin)s-based radiolabeling precursors were prepared in a one-step synthesis with chemical yields of 49-99%. Radiolabeling of the aryl-boronic esters with fluorine-18 or iodine-123 via CMRH furnished the corresponding radiolabeled arenes with RCC of 7-99% and 10-99%, respectively. Notably, a radiohalogenated prosthetic group containing a vinyl sulfone motif was obtained with an activity yield (AY) of 18 ± 3%, and applied towards the preparation of two clinically relevant PET tracers. Conclusions: This approach enables the synthesis of stable radiolabeling precursors and thus provides increased versatility in the application of CMRH, thereby supporting the development of novel PET and SPECT radiotracers.
Keywords: 123I-iodination; 18F-fluorination; copper-mediated radiohalogenation (CMRH); positron emission tomography (PET); prosthetic group; radiohalogenation; single-photon emission computed tomography (SPECT).
Conflict of interest statement
Austin Craig, Frederik Sachse, and Markus Laube are co-inventors for a patent application based on this manuscript’s findings submitted to the German Patent Office (PCT/DE2025/100397). Rotop Radiopharmacy GmbH has no role or interest in the work presented in this manuscript.
Figures


References
-
- Alavi A., Werner T.J., Stępień E.Ł., Moskal P. Unparalleled and Revolutionary Impact of PET Imaging on Research and Day to Day Practice of Medicine. Bio-Algorithms Med-Syst. 2022;17:203–212. doi: 10.1515/bams-2021-0186. - DOI
-
- Israel O., Pellet O., Biassoni L., De Palma D., Estrada-Lobato E., Gnanasegaran G., Kuwert T., La Fougère C., Mariani G., Massalha S., et al. Two Decades of SPECT/CT—The Coming of Age of a Technology: An Updated Review of Literature Evidence. Eur. J. Nucl. Med. Mol. Imaging. 2019;46:1990–2012. doi: 10.1007/s00259-019-04404-6. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

