Inhalable Nanotechnology-Based Drug Delivery Systems for the Treatment of Inflammatory Lung Diseases
- PMID: 40733101
- PMCID: PMC12298333
- DOI: 10.3390/pharmaceutics17070893
Inhalable Nanotechnology-Based Drug Delivery Systems for the Treatment of Inflammatory Lung Diseases
Abstract
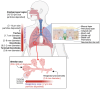
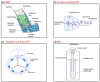
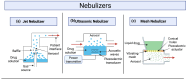
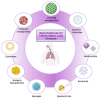
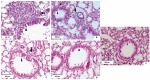
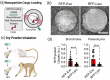
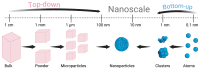
This review explores recent advancements in inhaled nanoparticle formulations and inhalation devices, with a focus on various types of nanoparticles used for inhalation to treat inflammatory lung diseases and the types of devices used in their delivery. Medical nebulizers have been found to be the most appropriate type of inhalation devices for the pulmonary delivery of nanoparticles, since formulations can be prepared using straightforward techniques, with no need for liquefied propellants as in the case of pressurized metered dose inhalers (pMDIs), or complicated preparation procedures as in the case of dry powder inhalers (DPIs). We demonstrated examples of how formulations should be designed considering the operation mechanism of nebulizers, and how an interplay of factors can affect the aerosol characteristics of nanoparticle formulations. Overall, nanoparticle-based formulations offer promising potential for the treatment of inflammatory lung diseases due to their unique physicochemical properties and ability to provide localized drug delivery in the lung following inhalation.
Keywords: drug delivery; inflammatory lung disorders; inhalation devices; nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- WHO The Top 10 Causes of Death. [(accessed on 8 January 2025)]. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
-
- Stern J., Pier J., Litonjua A.A. Seminars in Immunopathology. Springer; Berlin/Heidelberg, Germany: 2020. Asthma epidemiology and risk factors; pp. 5–15. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

