Trends and Commonalities of Approved and Late Clinical-Phase RNA Therapeutics
- PMID: 40733111
- PMCID: PMC12300435
- DOI: 10.3390/pharmaceutics17070903
Trends and Commonalities of Approved and Late Clinical-Phase RNA Therapeutics
Abstract
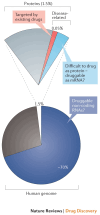
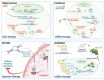
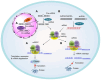
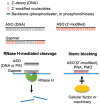
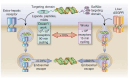
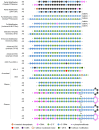
Background/Objectives: After many years of research and the successful development of therapeutic products by a few industrial actors, the COVID-19 vaccines brought messenger RNAs, as well as other nucleic acid modalities, such as antisense oligonucleotides, small interfering RNA, and aptamers, into the spotlight, eliciting renewed interest from both academia and industry. However, owing to their structure, relative "fragility", and the (usually) intracellular site of action, the delivery of these therapeutics has frequently proven to be a key limitation, especially when considering endosomal escape, which still needs to be overcome. Methods: By compiling delivery-related data on approved and late clinical-phase ribonucleic acid therapeutics, this review aims to assess the delivery strategies that have proven to be successful or are emerging, as well as areas where more research is needed. Results: In very specific cases, some strategies appeared to be quite effective, such as the N-acetylgalactosamine moiety in the case of liver delivery. Surprisingly, it also appears that for some modalities, efforts in molecular design have led to more "drug-like" properties, enablingthe administration of naked nucleic acids, without any form of encapsulation. This appears to be especially true when local administration, i.e., by injection, is possible, as this provides de facto targeting and a high local concentration, which can compensate for the small proportion of nucleic acids that reach the cytoplasm. Conclusions: Nucleic acid-based therapeutics have come a long way in terms of their physicochemical properties. However, due to their inherent limitations, targeting appears to be crucial for their efficacy, even more so than for traditional pharmaceutical modalities.
Keywords: delivery; endosomal escape; local administration; nucleic acids; targeting; topical.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Newey S. ‘No Limits’ to Possibilities of MRNA, Says Mastermind behind Technology. The Telegraph. Dec 29, 2022.
Publication types
LinkOut - more resources
Full Text Sources

