In Situ Targeting RGD-Modified Cyclodextrin Inclusion Complex/Hydrogel Hybrid System for Enhanced Glioblastoma Therapy
- PMID: 40733145
- PMCID: PMC12298666
- DOI: 10.3390/pharmaceutics17070938
In Situ Targeting RGD-Modified Cyclodextrin Inclusion Complex/Hydrogel Hybrid System for Enhanced Glioblastoma Therapy
Abstract
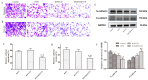
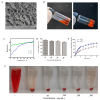
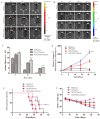
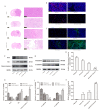
Background/Objectives: Glioblastoma (GBM) remains the most aggressive primary brain tumor, characterized by high malignancy, recurrence rate, and dismal prognosis, thereby demanding innovative therapeutic strategies. In this study, we report a novel in situ targeting inclusion complex hydrogel hybrid system (DOX/RGD-CD@Gel) that integrates doxorubicin (DOX) with RGD-conjugated cyclodextrin (RGD-CD) and a thermosensitive hydrogel for enhanced GBM therapy. Methods: The DOX/RGD-CD@Gel system was prepared by conjugating doxorubicin (DOX) with RGD-modified cyclodextrin (RGD-CD) and embedding it into a thermosensitive hydrogel. The drug delivery and antitumor efficacy of this system were evaluated in vitro and in vivo. Results: In vitro and in vivo evaluations demonstrated that DOX/RGD-CD@Gel significantly enhanced cytotoxicity compared to free DOX or DOX/CD formulations. The targeted delivery system effectively promoted apoptosis and inhibited cell proliferation and metastasis in GBM cells. Moreover, the hydrogel-based system exhibited prolonged drug retention in the brain, as evidenced by its temperature- and pH-responsive release characteristics. In a GBM mouse model, DOX/RGD-CD@Gel significantly suppressed tumor growth and improved survival rates. Conclusions: This study presents a paradigm of integrating a targeted inclusion complex with a thermosensitive hydrogel, offering a safe and efficacious strategy for localized GBM therapy with potential translational value.
Keywords: cyclodextrin; doxorubicin; glioblastoma; hydrogel; inclusion complex.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures




Similar articles
-
Exploration of RGD peptide-modified targeted delivery of doxorubicin using chitosan oligosaccharide-coated GQD carriers with different complexity.Int J Biol Macromol. 2025 Aug;320(Pt 2):145734. doi: 10.1016/j.ijbiomac.2025.145734. Epub 2025 Jul 7. Int J Biol Macromol. 2025. PMID: 40633866
-
Deciphering pericyte-induced temozolomide resistance in glioblastoma with a 3D microphysiological system mimicking the biomechanical properties of brain tissue.Acta Biomater. 2025 Jun 15;200:202-217. doi: 10.1016/j.actbio.2025.05.038. Epub 2025 May 16. Acta Biomater. 2025. PMID: 40383349
-
Nanobody-functionalized liposomal doxorubicin: A novel strategy for angiogenesis suppression via VEGFR2 targeting.Bioimpacts. 2025 Apr 6;15:30707. doi: 10.34172/bi.30707. eCollection 2025. Bioimpacts. 2025. PMID: 40584910 Free PMC article.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
CAR-T Cells Therapy in Glioblastoma: A Systematic Review on Molecular Targets and Treatment Strategies.Int J Mol Sci. 2024 Jun 29;25(13):7174. doi: 10.3390/ijms25137174. Int J Mol Sci. 2024. PMID: 39000281 Free PMC article.
References
-
- Fine H.A. Glioblastoma: Not Just Another Cancer. Cancer Discov. 2024;14:648–652. doi: 10.1158/2159-8290.CD-23-1498. - DOI - PubMed
-
- Obrador E., Moreno-Murciano P., Oriol-Caballo M., Lopez-Blanch R., Pineda B., Gutierrez-Arroyo J.L., Loras A., Gonzalez-Bonet L.G., Martinez-Cadenas C., Estrela J.M., et al. Glioblastoma Therapy: Past, Present and Future. Int. J. Mol. Sci. 2024;25:2529. doi: 10.3390/ijms25052529. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

