ROS/Enzyme Dual-Responsive Drug Delivery System for Targeted Colorectal Cancer Therapy: Synergistic Chemotherapy, Anti-Inflammatory, and Gut Microbiota Modulation
- PMID: 40733148
- PMCID: PMC12300498
- DOI: 10.3390/pharmaceutics17070940
ROS/Enzyme Dual-Responsive Drug Delivery System for Targeted Colorectal Cancer Therapy: Synergistic Chemotherapy, Anti-Inflammatory, and Gut Microbiota Modulation
Abstract
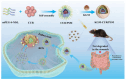
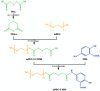
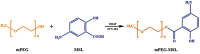
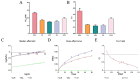
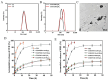
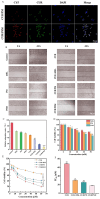
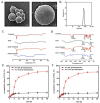
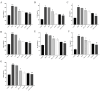
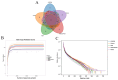
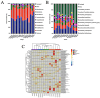
Objectives: Colorectal cancer (CRC) is a leading cause of cancer-related mortality, driven by chronic inflammation, gut microbiota dysbiosis, and complex tumor microenvironment interactions. Current therapies are limited by systemic toxicity and poor tumor accumulation. This study aimed to develop a ROS/enzyme dual-responsive oral drug delivery system, KGM-CUR/PSM microspheres, to achieve precise drug release in CRC and enhance tumor-specific drug accumulation, which leverages high ROS levels in CRC and the β-mannanase overexpression in colorectal tissues. Methods: In this study, we synthesized a ROS-responsive prodrug polymer (PSM) by conjugating polyethylene glycol monomethyl ether (mPEG) and mesalazine (MSL) via a thioether bond. CUR was then encapsulated into PSM using thin-film hydration to form tumor microenvironment-responsive micelles (CUR/PSM). Subsequently, konjac glucomannan (KGM) was employed to fabricate KGM-CUR/PSM microspheres, enabling targeted delivery for colorectal cancer therapy. The ROS/enzyme dual-response properties were confirmed through in vitro drug release studies. Cytotoxicity, cellular uptake, and cell migration were assessed in SW480 cells. In vivo efficacy was evaluated in AOM/DSS-induced CRC mice, monitoring tumor growth, inflammatory markers (TNF-α, IL-1β, IL-6, MPO), and gut microbiota composition. Results: In vitro drug release studies demonstrated that KGM-CUR/PSM microspheres exhibited ROS/enzyme-responsive release profiles. CUR/PSM micelles demonstrated significant anti-CRC efficacy in cytotoxicity assays, cellular uptake studies, and cell migration assays. In AOM/DSS-induced CRC mice, KGM-CUR/PSM microspheres significantly improved survival and inhibited CRC tumor growth, and effectively reduced the expression of inflammatory cytokines (TNF-α, IL-1β, IL-6) and myeloperoxidase (MPO). Histopathological and microbiological analyses revealed near-normal colon architecture and microbial diversity in the KGM-CUR/PSM group, confirming the system's ability to disrupt the "inflammation-microbiota-tumor" axis. Conclusions: The KGM-CUR/PSM microspheres demonstrated a synergistic enhancement of anti-tumor efficacy by inducing apoptosis, alleviating inflammation, and modulating the intestinal microbiota, which offers a promising stimuli-responsive drug delivery system for future clinical treatment of CRC.
Keywords: ROS/enzyme dual-responsive; colorectal cancer; gut microbiota; inflammations; microsphere; prodrug micelles; synergistic treatment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Almeida A., Castro F., Resende C., Lúcio M., Schwartz S., Jr., Sarmento B. Oral delivery of camptothecin-loaded multifunctional chitosan-based micelles is effective in reduce colorectal cancer. J. Control. Release Off. J. Control. Release Soc. 2022;349:731–743. doi: 10.1016/j.jconrel.2022.07.029. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

