Functional Role of Resveratrol in Inducing Apoptosis in Breast Cancer Subtypes via Inhibition of Intracellular Fatty Acid Synthase
- PMID: 40733158
- PMCID: PMC12298780
- DOI: 10.3390/molecules30142891
Functional Role of Resveratrol in Inducing Apoptosis in Breast Cancer Subtypes via Inhibition of Intracellular Fatty Acid Synthase
Abstract
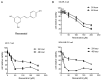
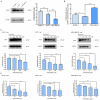
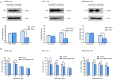
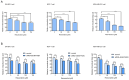
Fatty acid synthase (FASN) is frequently overexpressed in human breast cancer and has emerged as a potential therapeutic target. Resveratrol has been shown to inhibit FASN activity in vitro through both fast-reversible and slow-irreversible mechanisms. In this study, resveratrol reduced intracellular fatty acid levels by inhibiting FASN activity and downregulating its expression across various breast cancer subtypes, including SK-BR-3, MCF-7, and MDA-MB-231 cells. Knockdown of FASN via small interfering RNA (siRNA) further enhanced resveratrol-induced cytotoxicity. Resveratrol significantly suppressed cell viability and triggered apoptosis, as evidenced by increased cleavage of poly(ADP-ribose) polymerase (PARP) and disruption of Bcl-2 family protein balance. Furthermore, resveratrol inhibited key signaling pathways involved in cell proliferation and survival, notably FAK, AKT, and ERK1/2. FASN silencing by siRNA also modulated the activation states of these signaling proteins. Collectively, these findings support resveratrol as a promising anti-cancer candidate that induces apoptosis in diverse breast cancer subtypes via FASN inhibition.
Keywords: breast cancer cells; cell apoptosis; fatty acid synthase; inhibitor; resveratrol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Loss of fatty acid synthase suppresses the malignant phenotype of colorectal cancer cells by down-regulating energy metabolism and mTOR signaling pathway.J Cancer Res Clin Oncol. 2016 Jan;142(1):59-72. doi: 10.1007/s00432-015-2000-8. Epub 2015 Jun 25. J Cancer Res Clin Oncol. 2016. PMID: 26109148 Free PMC article.
-
Inhibition of fatty acid synthase suppresses neovascularization via regulating the expression of VEGF-A in glioma.J Cancer Res Clin Oncol. 2016 Dec;142(12):2447-2459. doi: 10.1007/s00432-016-2249-6. Epub 2016 Sep 6. J Cancer Res Clin Oncol. 2016. PMID: 27601165 Free PMC article.
-
Fatty acid synthase (FASN) inhibition cooperates with BH3 mimetic drugs to overcome resistance to mitochondrial apoptosis in pancreatic cancer.Neoplasia. 2025 Apr;62:101143. doi: 10.1016/j.neo.2025.101143. Epub 2025 Feb 24. Neoplasia. 2025. PMID: 39999714 Free PMC article.
-
Alpha-mangostin inhibits intracellular fatty acid synthase and induces apoptosis in breast cancer cells.Mol Cancer. 2014 Jun 3;13:138. doi: 10.1186/1476-4598-13-138. Mol Cancer. 2014. PMID: 24894151 Free PMC article.
-
Resveratrol in oral cancer: a systematic review of preclinical studies on its anticancer mechanisms and therapeutic potential.Med Oncol. 2025 Jul 13;42(8):329. doi: 10.1007/s12032-025-02903-1. Med Oncol. 2025. PMID: 40653555
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

