Geographic Influence and Metabolomics-Driven Discovery of 5-Alpha Reductase Inhibitors in Tectona grandis L.f. (Teak) Leaves
- PMID: 40733162
- PMCID: PMC12300835
- DOI: 10.3390/molecules30142895
Geographic Influence and Metabolomics-Driven Discovery of 5-Alpha Reductase Inhibitors in Tectona grandis L.f. (Teak) Leaves
Abstract
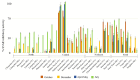
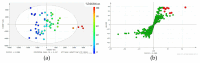
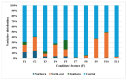
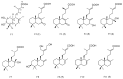
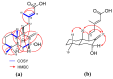
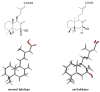
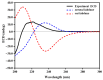
The inhibition of steroid 5-alpha reductase (S5AR), a key mechanism for managing dihydrotestosterone-dependent conditions, has been demonstrated in teak (Tectona grandis L.f.) leaf extracts. Our recent clinical study confirmed the effectiveness of a hair growth formulation containing teak leaf extract in males with androgenic alopecia. However, significant variability in S5AR inhibitory activity among teak leaf samples from different regions underscores the need for quality control of raw materials. This study applied a metabolomics approach to investigate the influence of leaf age, harvesting period, and geographic origin on chemical composition and S5AR inhibitory activity, as well as to identify active S5AR inhibitors. Geographic origin emerged as the primary determinant of variations in chemical profiles and S5AR inhibitory activity. Using orthogonal partial least squares analysis, six diterpenoid S5AR inhibitors were identified, including four compounds reported for the first time as S5AR inhibitors: rhinocerotinoic acid, 7-oxo-8-labden-15-oic acid, 8-hydroxy-labd-13-en-15-oic acid, and a novel diterpene, 7-hydroxy-labd-8,13-dien-15-oic acid. These findings highlight the potential of metabolomics as a powerful tool for discovering bioactive compounds and optimizing raw material selection. By prioritizing proven geographic sources, consistent bioactivity can be achieved, supporting the therapeutic potential of teak leaves in managing S5AR-related conditions.
Keywords: Tectona grandis L.f.; metabolomics; steroid 5-alpha reductase inhibitor; structure elucidation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Effectiveness and Safety of Hair Growth Formulation Containing Tectona grandis L.f (Teak) Leaf Extract: A Randomized, Double-Blind, Placebo-Controlled Study on Males with Androgenic Alopecia.J Evid Based Integr Med. 2024 Jan-Dec;29:2515690X241291141. doi: 10.1177/2515690X241291141. J Evid Based Integr Med. 2024. PMID: 39474646 Free PMC article. Clinical Trial.
-
Potential of Pandan Root and Teak Leaf Extracts in Managing Maternal Hyperglycemia During Pregnancy: Comparative Efficacy and Mechanistic Insights.Int J Mol Sci. 2025 Jun 9;26(12):5506. doi: 10.3390/ijms26125506. Int J Mol Sci. 2025. PMID: 40564970 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Isolation and HPLC Quantitative Determination of 5α-Reductase Inhibitors from Tectona grandis L.f. Leaf Extract.Molecules. 2022 Apr 30;27(9):2893. doi: 10.3390/molecules27092893. Molecules. 2022. PMID: 35566245 Free PMC article.
-
[Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data].Epidemiol Prev. 2013 Mar-Jun;37(2-3 Suppl 2):1-100. Epidemiol Prev. 2013. PMID: 23851286 Italian.
References
-
- Dhurat R., Sharma A., Rudnicka L., Kroumpouzos G., Kassir M., Galadari H., Wollina U., Lotti T., Golubovic M., Binic I. 5-Alpha reductase inhibitors in androgenetic alopecia: Shifting paradigms, current concepts, comparative efficacy, and safety. Dermatol. Ther. 2020;33:e13379. doi: 10.1111/dth.13379. - DOI - PubMed
-
- Fachrunniza Y., Srivilai J., Wisuitiprot V., Wisuitiprot W., Suphrom N., Temkitthawon P., Waranuch N., Ingkaninan K. Tectona grandis, a potential active ingredient for hair growth promotion. Songklanakarin J. Sci. Technol. 2020;42:1352–1359. doi: 10.14456/sjst-psu.2020.175. - DOI
MeSH terms
Substances
Grants and funding
- B16F640099/National Science, Research and 504 Innovation Fund (NSRF) via the Program Management Unit for Human Resources & Institutional Development, Research and Innovation
- Ministry of Higher Education, Science, Research and Innovation (MHESI) via Reinventing University Program 2023
- R2566C053/Global and Frontier Research University Fund, Naresuan University
- National Research Council of Thailand
- 2564/1/Agricultural Research Development Agency
LinkOut - more resources
Full Text Sources
Research Materials

