Halogen Migration in the Photofragmentation of Halothane
- PMID: 40733169
- PMCID: PMC12299474
- DOI: 10.3390/molecules30142902
Halogen Migration in the Photofragmentation of Halothane
Abstract
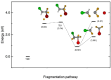
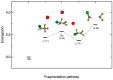
The photofragmentation of halothane (CF3CHBrCl) was studied with synchrotron radiation by photoionization efficiency (PIE) measurements and photoelectron-photoion coincidence (PEPICO) experiments, as well as by a theoretical exploration of potential energy surfaces. Among the other fragments, the formation of the CHClF+ and CHBrF+ ions, which involves the transfer of a F atom between the two moieties of the parent molecule, was observed. To understand the mechanisms leading to the halogen migration, a detailed theoretical study of the production of CHClF+, m/z 67+, based on DFT calculations and natural bond orbital (NBO) analysis was conducted. The results contribute to the understanding of the photochemistry of halothane, its polluting behavior in the high atmosphere, and the formation of highly reactive species.
Keywords: appearance energy; halogen migration; halothane; photofragmentation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










Similar articles
-
Effects of sevoflurane versus other general anaesthesia on emergence agitation in children.Cochrane Database Syst Rev. 2014 Sep 12;2014(9):CD007084. doi: 10.1002/14651858.CD007084.pub2. Cochrane Database Syst Rev. 2014. PMID: 25212274 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Theoretical Insights into Halogen Substitution Effects on Room Temperature Phosphorescence in Twisted Halogenated Tetraphenylene Derivatives.J Phys Chem A. 2025 Jun 19;129(24):5267-5280. doi: 10.1021/acs.jpca.5c01904. Epub 2025 Jun 6. J Phys Chem A. 2025. PMID: 40474703
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Interventions for patients and caregivers to improve knowledge of sickle cell disease and recognition of its related complications.Cochrane Database Syst Rev. 2016 Oct 6;10(10):CD011175. doi: 10.1002/14651858.CD011175.pub2. Cochrane Database Syst Rev. 2016. PMID: 27711980 Free PMC article.
References
-
- Andersen M.P.S., Nielsen O.J., Sherman J.D. Assessing the potential climate impact of anaesthetic gases. Lancet Planet. Health. 2023;7:e622–e629. - PubMed
-
- Shiraishi Y., Ikeda K. Uptake and biotransformation of sevoflurane in humans: A comparative study of sevof lurane with halothane, enflurane, and isoflurane. J. Clin. Anesth. 1990;2:381–386. - PubMed
-
- Brown A.C., Canosa-Mas C.E., Parr A.D., Pierce J.M.T., Waine R.P. Tropospheric lifetimes of halogenated anaesthetics. Nature. 1989;341:635–637. - PubMed
-
- Langbein T., Sonntag H., Trapp D., Hoffmann A., Malms W., Roth E.-P., Mörs V., Zellner R. Volatile anaesthetics and the atmosphere: Atmospheric lifetimes and atmospheric effects of halothane, enflurane, isoflurane, desflurane and sevoflurane. Br. J. Anaesth. 1999;82:66–73. - PubMed
-
- Ferreira da Silva F., Duflot D., Hoffmann S.V., Jones N.C., Rodrigues F.N., Ferreira-Rodriguez A.M., de Souza G.G.B., Mason N.J., Eden S., Limao-Vieira P. Electronic State Spectroscopy of Halothane As Studied by ab Initio Calculations, Vacuum Ultraviolet Synchrotron Radiation, and Electron Scattering Methods. J. Phys. Chem. A. 2015;119:8503–8511. - PubMed
Grants and funding
- PGR 00220/Italian Ministry of Foreign Affairs and International Cooperation,
- PGR02090/Italian Ministry of Foreign Affairs and International Cooperation
- CUP I93C21000160006, IR0000030/PNRR-IR project EuPRAXIA
- VSC (Flemish Supercomputer Center)/Research Foundation-Flanders and the Flemish Government
- EUROFEL-ROADMAP ESFRI/Italian Ministry of University and Research
LinkOut - more resources
Full Text Sources

