Pyrrolopyrimidines: Design, Synthesis and Antitumor Properties of Novel Tricyclic Pyrrolo [2,3- d]pyrimidine Derivatives
- PMID: 40733183
- PMCID: PMC12299203
- DOI: 10.3390/molecules30142917
Pyrrolopyrimidines: Design, Synthesis and Antitumor Properties of Novel Tricyclic Pyrrolo [2,3- d]pyrimidine Derivatives
Abstract
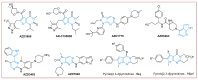
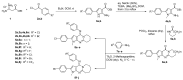
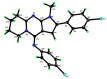
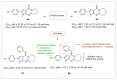
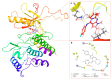
The pyrrolo[2,3-d]pyrimidine (7-deazapurine) scaffold is a unique heterocyclic system included in the composition of most nucleotides. In this study, series of the pyrrolo[2,3-d]pyrimidine-imines and 3-halo-substituted pyrrolo[2,3-d]pyrimidines were designed and prepared in high yields. Condensed pyrimidines are obtained via carbonyl-amine condensation and carbon-halogen bond formation. Pyrrolo[2,3-d]pyrimidine-imines containing a bromine substituent at position C-4 of the phenyl ring and azepine side-ring exhibited superior antitumor activity on the colon cancer HT-29 cell line; IC50 values were 4.55 and 4.01 µM, respectively. These results revealed an interesting pattern, where condensed pyrimidinones containing an azepine ring demonstrated selective antitumor activity on the colon cancer cell line HT-29. In addition, the molecular docking results suggest that compound 8g provided a thorough understanding of its interactions with the DDR2 active site. This could pave the way for further development and optimization of DDR-targeting drugs, contributing to advancements in cancer therapeutics. This lead compound may serve as design templates for further studies.
Keywords: 7-deazapurine; DDR2 receptor; HT-29 cell line; N-halosuccinimide; Tf2O; antitumor activity; carbonyl-amine condensation; molecular docking; pyrrolo[2,3-d]pyrimidine; pyrrolo[2,3-d]pyrimidine-imines.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Ruzi Z., Bozorov K., Nie L., Zhao J., Akber Aisa H. Discovery of novel (E)-1-methyl-9-(3-methylbenzylidene)-6,7,8,9-tetrahydropyrazolo[3,4-d]pyrido[1,2-a]pyrimidin-4(1H)-one as DDR2 kinase inhibitor: Synthesis, molecular docking, and anticancer properties. Bioorg. Chem. 2023;135:106506. doi: 10.1016/j.bioorg.2023.106506. - DOI - PubMed
-
- Murtazaeva Z., Nasrullaev A., Buronov A., Gaybullaev S., Nie L., Numonov S., Khushnazarov Z., Turgunov D., Kuryazov R., Zhao J., et al. Imidazole Hybrids: A Privileged Class of Heterocycles in Medicinal Chemistry with New Insights into Anticancer Activity. Molecules. 2025;30:2245. doi: 10.3390/molecules30102245. - DOI - PMC - PubMed
-
- Beloueche-Babari M., Casals Galobart T., Delgado-Goni T., Wantuch S., Parkes H.G., Tandy D., Harker J.A., Leach M.O. Monocarboxylate transporter 1 blockade with AZD3965 inhibits lipid biosynthesis and increases tumour immune cell infiltration. Br. J. Cancer. 2020;122:895–903. doi: 10.1038/s41416-019-0717-x. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- (No. 442 2020YFE0205600/National Key R&D Program of China
- No. 443 NBSDC-DB-19/CAM Resources Data Base" in the National Basic Science Data Center
- No. ALM-444 202310062530/Organization of the laboratory for the creation of anticancer drugs
- No. 2024VBA0021/Chinese Academy of Sciences President's International Fellowship 445 Initiative
LinkOut - more resources
Full Text Sources
Miscellaneous

