IrO2-Decorated Titania Nanotubes as Oxygen Evolution Anodes
- PMID: 40733188
- PMCID: PMC12300787
- DOI: 10.3390/molecules30142921
IrO2-Decorated Titania Nanotubes as Oxygen Evolution Anodes
Abstract
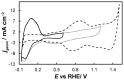
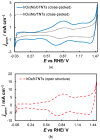
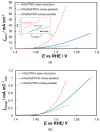
In this work, we have used both plain titania nanotubes, TNTs, and their reduced black analogues, bTNTs, that bear metallic conductivity (prepared by solid state reaction of TNTs with CaH2 at 500 °C for 2 h), as catalyst supports for the oxygen evolution reaction (OER). Ir was subsequently been deposited on them by the galvanic replacement of electrodeposited Ni by Ir(IV) chloro-complexes; this was followed by Ir electrochemical anodization to IrO2. By carrying out the preparation of the TNTs in either two or one anodization steps, we were able to produce close-packed or open-structure nanotubes, respectively. In the former case, larger than 100 nm Ir aggregates were finally formed on the top face of the nanotubes (leading to partial or full surface coverage); in the latter case, Ir nanoparticles smaller than 100 nm were obtained, with some of them located inside the pores of the nanotubes, which retained a porous surface structure. The electrocatalytic activity of IrO2 supported on open-structure bTNTs towards OER is superior to that supported on close-packed bTNTs and TNTs, and its performance is comparable or better than that of similar electrodes reported in the literature (overpotential of η = 240 mV at 10 mA cm-2; current density of 70 mA cm-2 and mass specific current density of 258 mA mgIr-1 at η = 300 mV). Furthermore, these electrodes demonstrated good medium-term stability, maintaining stable performance for 72 h at 10 mA cm-2 in acid.
Keywords: acid water electrolysis; black titanium dioxide; dimensionally stable anodes; galvanic replacement; iridium oxide; titanium dioxide nanotubes.
Conflict of interest statement
Author Patricia Carvahlo was employed by the company SINTEF. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
References
-
- Duby P. The History of Progress in Dimensionally Stable Anodes. JOM. 1993;45:41–43. doi: 10.1007/BF03222350. - DOI
-
- Pavlović M.G., Dekanski A. On the Use of Platinized and Activated Titanium Anodes in Some Electrodeposition Processes. J. Solid. State Electrochem. 1997;1:208–214. doi: 10.1007/s100080050050. - DOI
-
- Zhang W., Ghali E., Houlachi G. Review of Oxide Coated Catalytic Titanium Anodes Performance for Metal Electrowinning. Hydrometallurgy. 2017;169:456–467. doi: 10.1016/j.hydromet.2017.02.014. - DOI
-
- Yasutake M., Kawachino D., Noda Z., Matsuda J., Lyth S.M., Ito K., Hayashi A., Sasaki K. Catalyst-Integrated Gas Diffusion Electrodes for Polymer Electrolyte Membrane Water Electrolysis: Porous Titanium Sheets with Nanostructured TiO2 Surfaces Decorated with Ir Electrocatalysts. J. Electrochem. Soc. 2020;167:124523. doi: 10.1149/1945-7111/abb37d. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

