Design, Synthesis, and Study of Protective Activity Against Stroke for Novel Water-Soluble Aldehyde Dehydrogenase 2 Activators
- PMID: 40733191
- PMCID: PMC12301025
- DOI: 10.3390/molecules30142924
Design, Synthesis, and Study of Protective Activity Against Stroke for Novel Water-Soluble Aldehyde Dehydrogenase 2 Activators
Abstract
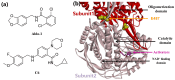
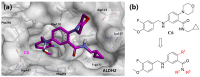
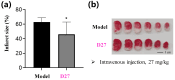
Stroke poses a serious threat to human health, while there are very few drugs that can directly alleviate ischemia/reperfusion injury and improve the prognosis. Studies have shown that small-molecule activators of aldehyde dehydrogenase 2 (ALDH2) have the potential to become novel therapeutic drugs for ischemic stroke. In this study, through the systematic structural optimization of novel N-benzylaniline-based ALDH2 activators obtained from our previous virtual screening, ALDH2 activators with improved water solubility and activity were obtained. Among them, compound D10 exhibits the best activity, with a maximum activation fold reaching 114% relative to Alda-1. And the water solubility of its hydrochloride salt D27 was increased by more than 200-fold. The intravenous injection of this compound can significantly reduce the infarct area in the rat model of cerebral infarction compared with the model group. This study lays a good foundation for the future research on ALDH2 activators used in the treatment of stroke.
Keywords: activators; aldehyde dehydrogenase 2; cerebral infarction; stroke; water-soluble.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
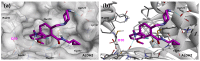

Similar articles
-
Remote Ischemic Postconditioning Improve Cerebral Ischemia-Reperfusion Injury Induced Cognitive Dysfunction through Suppressing Mitochondrial Apoptosis in Hippocampus via TK/BK/B2R-Mediated PI3K/AKT.Mol Neurobiol. 2025 Aug;62(8):10652-10669. doi: 10.1007/s12035-025-04864-y. Epub 2025 Apr 14. Mol Neurobiol. 2025. PMID: 40229456 Free PMC article.
-
Design, Synthesis, and Protective Effect Evaluation on Myocardial Ischemia of New Triazole Aldehyde Dehydrogenase 2 Activators.ACS Med Chem Lett. 2025 Jun 9;16(7):1420-1427. doi: 10.1021/acsmedchemlett.5c00291. eCollection 2025 Jul 10. ACS Med Chem Lett. 2025. PMID: 40666449
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Thrombolysis for acute ischaemic stroke.Cochrane Database Syst Rev. 2003;(3):CD000213. doi: 10.1002/14651858.CD000213. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2009 Oct 07;(4):CD000213. doi: 10.1002/14651858.CD000213.pub2. PMID: 12917889 Updated.
References
-
- Wang L., editor. Report on the Prevention and Treatment of Stroke in China 2021. China Population Publishing House; Beijing, China: 2022.
-
- National Center for Cardiovascular Diseases, editor. Report on Cardiovascular Diseases in China 2023. Encyclopedia of China Publishing House; Beijing, China: 2024.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

