Insights from the Absorption Coefficient for the Development of Polarizable (Multipole) Force Fields
- PMID: 40733210
- PMCID: PMC12300768
- DOI: 10.3390/molecules30142941
Insights from the Absorption Coefficient for the Development of Polarizable (Multipole) Force Fields
Abstract
We present a detailed examination of the absorption coefficients in the THz region for different water models using different types of potentials: the non-polarizable SPC/E, the Drude-polarizable SWM4-NDP and OPC3-pol, IPOL-0.13 and the multipole AMOEBA14 water. The primary focus is on understanding the interplay between permanent and induced dipole moments and their influence on the THz spectrum. Although the induced dipoles strongly contribute to the peak at 200 cm-1, merely increasing the induced dipole moments does not improve the agreement with experiments. We aim to investigate the behavior of the intensity at 200 cm-1 depending on the water model. Furthermore, we dissect the THz spectra of the water models into distinct contributions to gain more insight into the inter- and intramolecular interactions. Intermolecular interactions significantly contribute to the low-frequency peak, while the peak observed at 600 cm-1 can be adequately attributed to intramolecular dipole-dipole interactions.
Keywords: computational spectroscopy; induced dipoles; terahertz spectroscopy; water.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


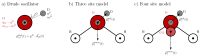



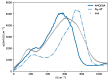

Similar articles
-
A new method to calculate broadband dielectric spectra of solvents from molecular dynamics simulations demonstrated with polarizable force fields.J Chem Phys. 2024 Aug 14;161(6):064306. doi: 10.1063/5.0217883. J Chem Phys. 2024. PMID: 39132799 Free PMC article.
-
Terahertz and Dielectric Spectroscopy of Aqueous MgCl2 Solutions: Self- and Cross-Correlation Contributions of Permanent and Induced Dipoles Using Polarizable Models.J Phys Chem B. 2025 Jul 10;129(27):6954-6964. doi: 10.1021/acs.jpcb.5c02574. Epub 2025 Jun 26. J Phys Chem B. 2025. PMID: 40569390
-
Unraveling the Anisotropy of Intermolecular Interactions of Water at the Liquid-Vapor Interface with Terahertz Sum Frequency Generation Spectroscopy Using a Polarizable Force Field.J Phys Chem B. 2025 Jun 19;129(24):6069-6077. doi: 10.1021/acs.jpcb.5c02610. Epub 2025 Jun 6. J Phys Chem B. 2025. PMID: 40479521
-
Falls prevention interventions for community-dwelling older adults: systematic review and meta-analysis of benefits, harms, and patient values and preferences.Syst Rev. 2024 Nov 26;13(1):289. doi: 10.1186/s13643-024-02681-3. Syst Rev. 2024. PMID: 39593159 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
References
-
- Zasetsky A.Y., Lileev A.S., Lyashchenko A.K. Molecular dynamic simulations of terahertz spectra for water-methanol mixtures. Mol. Phys. 2010;108:649–656. doi: 10.1080/00268971003657086. - DOI
-
- Cherkasova O., Nazarov M., Konnikova M., Shkurinov A. THz spectroscopy of bound water in glucose: Direct measurements from crystalline to dissolved state. J. Infrared Millim. 2020;41:1057–1068. doi: 10.1007/s10762-020-00684-4. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

