Pyridine-Quinoline and Biquinoline-Based Ruthenium p-Cymene Complexes as Efficient Catalysts for Transfer Hydrogenation Studies: Synthesis and Structural Characterization
- PMID: 40733211
- PMCID: PMC12300421
- DOI: 10.3390/molecules30142945
Pyridine-Quinoline and Biquinoline-Based Ruthenium p-Cymene Complexes as Efficient Catalysts for Transfer Hydrogenation Studies: Synthesis and Structural Characterization
Abstract
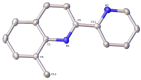
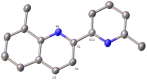
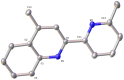
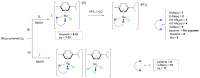
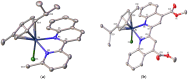
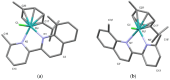
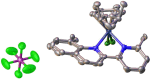
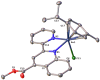
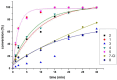
Searching for new and efficient transfer hydrogenation catalysts, a series of new organometallic ruthenium(II)-arene complexes of the formulae [Ru(η6-p-cymene)(L)Cl][PF6] (1-8) and [Ru(η6-p-cymene)(L)Cl][Ru(η6-p-cymene)Cl3] (9-11) were synthesized and fully characterized. These were prepared from the reaction of pyridine-quinoline and biquinoline-based ligands (L) with [Ru(η6-p-cymene)(μ-Cl)Cl]2, in 1:2 and 1:1, metal (M) to ligand (L) molar ratios. Characterization includes a combination of spectroscopic methods (FT-IR, UV-Vis, multi nuclear NMR), elemental analysis and single-crystal X-ray crystallography. The pyridine-quinoline organic entities encountered, were prepared in high yield either via the thermal decarboxylation of the carboxylic acid congeners, namely 2,2'-pyridyl-quinoline-4-carboxylic acid (pqca), 8-methyl-2,2'-pyridyl-quinoline-4-carboxylic acid (8-Mepqca), 6'-methyl-2,2'-pyridyl-quinoline-4-carboxylic acid (6'-Mepqca) and 8,6'-dimethyl-2,2'-pyridyl-quinoline-4-carboxylic acid (8,6'-Me2pqca), affording the desired ligands pq, 8-Mepq, 6'-Mepq and 8,6'-Me2pq, or by the classical Friedländer condensation, to yield 4,6'-dimethyl-2,2'-pyridyl-quinoline (4,6'-Me2pq) and 4-methyl-2,2'-pyridyl-quinoline (4-Mepq), respectively. The solid-state structures of complexes 1-4, 6, 8 and 9 were determined showing a distorted octahedral coordination geometry. The unit cell of 3 contains two independent molecules (Ru-3), (Ru'-3) in a 1:1 ratio, due to a slight rotation of the arene ring. All complexes catalyze the transfer hydrogenation of acetophenone, using 2-propanol as a hydrogen donor in the presence of KOiPr. Among them, complexes 1 and 5 bearing methyl groups at the 8 and 4 position of the quinoline moiety, convert acetophenone to 1-phenylethanol quantitatively, within approximately 10 min with final TOFs of 1600 h-1. The catalytic performance of complexes 1-11, towards the transfer hydrogenation of p-substituted acetophenone derivatives and benzophenone, ranges from moderate to excellent. An inner-sphere mechanism has been suggested based on the detection of ruthenium(II) hydride species.
Keywords: hydride species; hydrogenation; ketones; p-cymene; pyridine–quinoline ligands; ruthenium complexes; transfer hydrogenation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
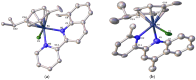

Similar articles
-
Ruthenium p-Cymene Complexes Incorporating Substituted Pyridine-Quinoline-Based Ligands: Synthesis, Characterization, and Cytotoxic Properties.Molecules. 2024 Jul 6;29(13):3215. doi: 10.3390/molecules29133215. Molecules. 2024. PMID: 38999167 Free PMC article.
-
Sterically demanding pyridine-quinoline anchoring ligands as building blocks for copper(I)-based dye-sensitized solar cell (DSSC) complexes.Dalton Trans. 2022 Oct 11;51(39):15049-15066. doi: 10.1039/d2dt02382b. Dalton Trans. 2022. PMID: 36112091
-
Enhanced Cytotoxicity of Half-Sandwich Ruthenium(II) Complex Containing Nitro-Substituted Salicyllimidazo[1,5-a]pyridine toward Hormone-Independent Triple-Negative Breast Cancer Cells.Inorg Chem. 2025 Jul 21;64(28):14073-14090. doi: 10.1021/acs.inorgchem.5c00090. Epub 2025 Jul 9. Inorg Chem. 2025. PMID: 40632111
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
References
-
- Blaser H.U., Malan C., Pugin B., Spindler F., Steiner H., Studer M. Selective Hydrogenation for Fine Chemicals: Recent Trends and New Developments. Adv. Synth. Catal. 2003;345:103–151. doi: 10.1002/adsc.200390000. - DOI
-
- Baidilov D., Hayrapetyan D., Khalimon A.Y. Recent advances in homogeneous base-metal-catalyzed transfer hydrogenation reactions. Tetrahedron. 2021;98:132435. doi: 10.1016/j.tet.2021.132435. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

