A Comprehensive Review of Radical-Mediated Intramolecular Cyano-Group Migration
- PMID: 40733223
- PMCID: PMC12298859
- DOI: 10.3390/molecules30142959
A Comprehensive Review of Radical-Mediated Intramolecular Cyano-Group Migration
Abstract
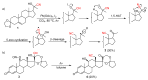
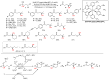
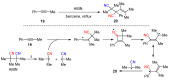
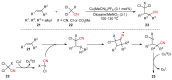
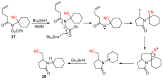
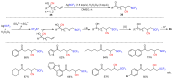
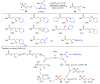
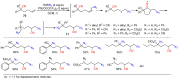
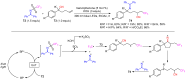
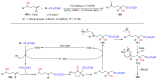
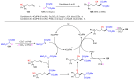
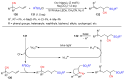
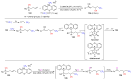
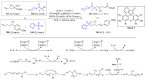
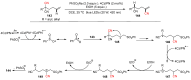
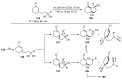
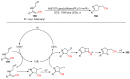
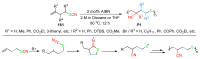
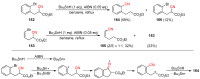
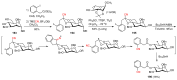
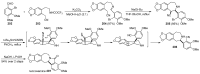
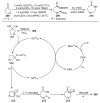
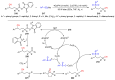
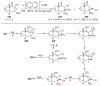
The radical-mediated intramolecular translocation of cyano groups has been recognized as a useful tool for the site-selective functionalization of organic molecules. The process is believed to proceed through the addition of an in situ-generated carbon-centered radical to the nitrile triple bond, followed by the β-scission of the resulting cyclic iminyl radical intermediate to relocate the cyano group and produce a more stable carbon radical for further elaboration. Beginning in the early 1960s and continuing for the next forty years, the research in this particular area has seen a surge of growth during the past two decades with advancements in radical chemistry and photocatalysis. The present article attempts to conduct a comprehensive review of existing studies on this topic by covering the literature from 1961 to 2025. The procedures developed for the purpose are grouped and discussed in four sections according to the strategies used to generate the initial carbon radicals, which include (i) hydrogen-atom transfer (HAT), (ii) radical addition to the π system, (iii) halogen-atom transfer (XAT), and (iv) the homolytic fission of a C-C single bond. In each section, a specific emphasis will be placed on reaction conditions, substrate scopes, and mechanisms.
Keywords: cyano group; intramolecular migration; nitrile; photocatalysis; radicals; site-selective functionalization; translocation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
































Similar articles
-
Hydrogen Atom Transfer Promoted by Carbon-Centered Biradicals via Energy Transfer Catalysis.Acc Chem Res. 2025 Jul 1;58(13):2028-2045. doi: 10.1021/acs.accounts.5c00228. Epub 2025 Jun 9. Acc Chem Res. 2025. PMID: 40490849
-
Light-Driven C(sp3)-C(sp3) Bond Functionalizations Enabled by the PCET Activation of Alcohol O-H Bonds.Acc Chem Res. 2025 Jul 1;58(13):2061-2071. doi: 10.1021/acs.accounts.5c00246. Epub 2025 Jun 13. Acc Chem Res. 2025. PMID: 40511721
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Seth A., Mehta P.K. In: The Chemistry of Nitriles. 1st ed. Seth A., editor. Nova Science Publishers, Inc.; Hauppauge, NY, USA: 2024.
-
- Xia Y., Jiang H., Wu W. Recent Advances in Chemical Modifications of Nitriles. Eur. J. Org. Chem. 2021:6658–6669. doi: 10.1002/ejoc.202101196. - DOI
-
- Harnett G.J., Hauck U., Hayes J.J., Hoffmann U., Lohri B., Meade M., Morawitz F., Plesniak M.P., Stahr H., Veits J., et al. Development and Demonstration of a High-Volume Manufacturing Process for a Key Intermediate of Dalcetrapib: Investigations on the Alkylation of Carboxylic Acids, Esters, and Nitriles. Org. Process Res. Dev. 2023;27:2329–2346. doi: 10.1021/acs.oprd.3c00304. - DOI
-
- Fan T., Qin J., Du X., Yuan F., Zhao H., Long J., Dong L., Chen J., Long Y., Ma J. Amorphous Fe0.7Cu0.3Ox as a Bifunctional Catalyst for Selective Hydration of Nitriles to Amides in Water. ACS Sustain. Chem. Eng. 2024;12:7791–7801. doi: 10.1021/acssuschemeng.4c00752. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

