Bioactive Cyclopeptide Alkaloids and Ceanothane Triterpenoids from Ziziphus mauritiana Roots: Antiplasmodial Activity, UHPLC-MS/MS Molecular Networking, ADMET Profiling, and Target Prediction
- PMID: 40733224
- PMCID: PMC12299143
- DOI: 10.3390/molecules30142958
Bioactive Cyclopeptide Alkaloids and Ceanothane Triterpenoids from Ziziphus mauritiana Roots: Antiplasmodial Activity, UHPLC-MS/MS Molecular Networking, ADMET Profiling, and Target Prediction
Abstract
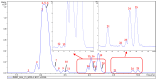
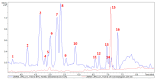
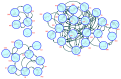
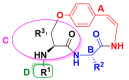
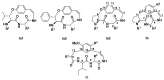
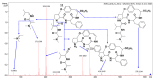
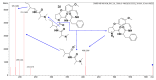
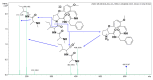
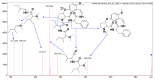
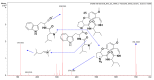
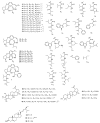
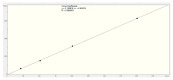
Malaria continues to pose a significant global health burden, driving the search for novel antimalarial agents to address emerging drug resistance. This study evaluated the antiplasmodial potential of Ziziphus mauritiana Lam. (Rhamnaceae) roots through an integrated phytochemical and pharmacological approach. The ethanol extract, along with its derived fractions, demonstrated potent in vitro activity against the chloroquine-sensitive Plasmodium falciparum strain 3D7 (Pf3D7), with the ethyl acetate-soluble (IC50 = 11.35 µg/mL) and alkaloid-rich (IC50 = 4.75 µg/mL) fractions showing particularly strong inhibition. UHPLC-DAD-ESI-QTOF-MS/MS-based molecular networking enabled the identification of thirty-two secondary metabolites (1-32), comprising twenty-five cyclopeptide alkaloids (CPAs), five of which had not yet been described (11, 20, 22, 23, 25), and seven known triterpenoids. Bioactivity-guided isolation yielded thirteen purified compounds (5, 6, 14, 26-30, 32-36), with betulinic acid (30; IC50 = 19.0 µM) and zizyberenalic acid (32; IC50 = 20.45 µM) exhibiting the most potent antiplasmodial effects. Computational ADMET analysis identified mauritine F (4), hemisine A (10), and nummularine R (21) as particularly promising lead compounds, demonstrating favourable pharmacokinetic properties, low toxicity profiles, and predicted activity against both family A G protein-coupled receptors and evolutionarily distinct Plasmodium protein kinases. Quantitative analysis revealed exceptionally high concentrations of key bioactive constituents, notably zizyberenalic acid (24.3 mg/g) in the root extracts. These findings provide robust scientific validation for the traditional use of Z. mauritiana in malaria treatment while identifying specific cyclopeptide alkaloids and triterpenoids as valuable scaffolds for antimalarial drug development. The study highlights the effectiveness of combining advanced metabolomics, bioassay-guided fractionation, and computational pharmacology in natural product-based drug discovery against resistant malaria strains.
Keywords: ADMET profiling; Ziziphus mauritiana; antiplasmodial activity; ceanothane triterpenoids; cyclopeptide alkaloids; in silico pharmacology.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
Antiplasmodial and antimycobacterial cyclopeptide alkaloids from the root of Ziziphus mauritiana.Phytochemistry. 2011 Jun;72(9):909-15. doi: 10.1016/j.phytochem.2011.03.003. Epub 2011 Mar 28. Phytochemistry. 2011. PMID: 21450320
-
Chemical profiling and quantification of potential active constituents responsible for the antiplasmodial activity of Cissampelos pareira.J Ethnopharmacol. 2020 Nov 15;262:113185. doi: 10.1016/j.jep.2020.113185. Epub 2020 Jul 26. J Ethnopharmacol. 2020. PMID: 32726676
-
Isolation and Structure Elucidation by LC-DAD-MS and LC-DAD-SPE-NMR of Cyclopeptide Alkaloids from the Roots of Ziziphus oxyphylla and Evaluation of Their Antiplasmodial Activity.J Nat Prod. 2016 Nov 23;79(11):2865-2872. doi: 10.1021/acs.jnatprod.6b00633. Epub 2016 Nov 10. J Nat Prod. 2016. PMID: 27933893
-
Primaquine or other 8-aminoquinoline for reducing P. falciparum transmission.Cochrane Database Syst Rev. 2014 Jun 30;(6):CD008152. doi: 10.1002/14651858.CD008152.pub3. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2015 Feb 19;(2):CD008152. doi: 10.1002/14651858.CD008152.pub4. PMID: 24979199 Free PMC article. Updated.
-
Primaquine or other 8-aminoquinoline for reducing Plasmodium falciparum transmission.Cochrane Database Syst Rev. 2015 Feb 19;(2):CD008152. doi: 10.1002/14651858.CD008152.pub4. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2018 Feb 02;2:CD008152. doi: 10.1002/14651858.CD008152.pub5. PMID: 25693791 Free PMC article. Updated.
References
-
- World Malaria Report 2024. [(accessed on 14 February 2025)]. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria....
-
- Asua V., Conrad M.D., Aydemir O., Duvalsaint M., Legac J., Duarte E., Tumwebaze P., Chin D., Cooper R., Yeka A., et al. Changing Prevalence of Potential Mediators of Aminoquinoline, Antifolate, and Artemisinin Resistance Across Uganda. J. Infect. Dis. 2021;223:985–994. doi: 10.1093/infdis/jiaa687. - DOI - PMC - PubMed
-
- Jha D., Hangargekar P., Akbar M., Parihar A.S., Kashyap S., Joshi A., Rahman M.A. Ziziphus mauritiana: An in-depth Review of its Medicinal Attributes and Pharmacological Activities. Intell. Pharm. 2024;2:274–283. doi: 10.1016/j.ipha.2023.12.001. - DOI
-
- Mishra T., Bhatia A. Antiplasmodial effects of the aqueous ethanolic seed extract of Ziziphus mauritiana against Plasmodium berghei in Swiss albino mice. Int. J. Pharmacol. Res. 2014;4:111–116.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

