Dual Inhibitors of Acetylcholinesterase and Monoamine Oxidase-B for the Treatment of Alzheimer's Disease
- PMID: 40733241
- PMCID: PMC12301006
- DOI: 10.3390/molecules30142975
Dual Inhibitors of Acetylcholinesterase and Monoamine Oxidase-B for the Treatment of Alzheimer's Disease
Abstract
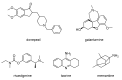
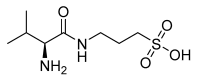
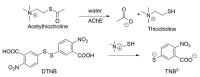
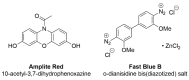
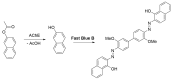
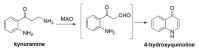
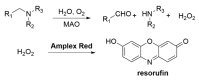
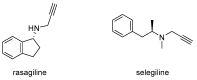
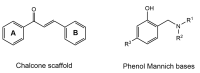
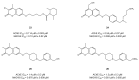
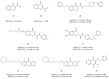
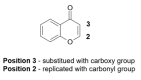
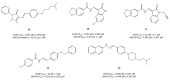
Alzheimer's disease (AD) is a multi-factorial neurodegenerative disease with a complex pathomechanism that can be best treated with multi-target medications. Among the possible molecular targets involved in AD, acetylcholinesterase (AChE) and monoamine oxidase B (MAO-B) are well recognized because they control the neurotransmitters responsible for memory processes. This review discusses the current understanding of AD pathology, recent advances in AD treatment, and recent reports in the field of dual AChE/MAO-B inhibitors for treating AD. We provide a classification of dual inhibitors based on their chemical structure and describe active compounds belonging to, i.a., chalcones, coumarins, chromones, imines, and hydrazones. Special emphasis is given to the computer-aided strategies of dual inhibitors design, their structure-activity relationships, and their interactions with the molecular targets at the molecular level.
Keywords: Acetylcholine Esterase (AChE); Monoamine Oxidase-B (MAO-B); SAR; dual inhibitors; molecular modeling.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


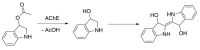
Similar articles
-
ML-based prediction to experimental validation: Development of dihydroquinazoline based multi-potent ligands as anti-Alzheimer's agents.Comput Biol Med. 2025 Sep;196(Pt A):110762. doi: 10.1016/j.compbiomed.2025.110762. Epub 2025 Jul 14. Comput Biol Med. 2025. PMID: 40664126
-
Dual inhibition of AChE and MAO-B in Alzheimer's disease: machine learning approaches and model interpretations.Mol Divers. 2025 Aug;29(4):3113-3130. doi: 10.1007/s11030-024-11061-x. Epub 2025 Jan 21. Mol Divers. 2025. PMID: 39838228
-
Novel Purine-Based Natural Products as Inhibitors of Cholinesterases and Monoamine Oxidases Presenting Potential Multitarget Therapeutics Tackling Alzheimer's Disease.Arch Pharm (Weinheim). 2025 Jul;358(7):e70035. doi: 10.1002/ardp.70035. Arch Pharm (Weinheim). 2025. PMID: 40629981
-
Latest advances in dual inhibitors of acetylcholinesterase and monoamine oxidase B against Alzheimer's disease.J Enzyme Inhib Med Chem. 2023 Dec;38(1):2270781. doi: 10.1080/14756366.2023.2270781. Epub 2023 Nov 13. J Enzyme Inhib Med Chem. 2023. PMID: 37955252 Free PMC article. Review.
-
Fighting Alzheimer's naturally: Peptides as multitarget drug leads.Bioorg Med Chem Lett. 2025 Nov 1;127:130305. doi: 10.1016/j.bmcl.2025.130305. Epub 2025 Jun 8. Bioorg Med Chem Lett. 2025. PMID: 40494420 Review.
References
-
- Andrade-Guerrero J., Santiago-Balmaseda A., Jeronimo-Aguilar P., Vargas-Rodríguez I., Cadena-Suárez A.R., Sánchez-Garibay C., Pozo-Molina G., Méndez-Catalá C.F., Cardenas-Aguayo M.-C., Diaz-Cintra S. Alzheimer’s Disease: An Updated Overview of Its Genetics. Int. J. Mol. Sci. 2023;24:3754. doi: 10.3390/ijms24043754. - DOI - PMC - PubMed
-
- Akyol M.A., Söylemez B.A., Küçükgüçlü Ö. Effects of Early-and Late-Onset Alzheimer’s Disease on Caregiver Burden. Turk. J. Fam. Med. Prim. Care. 2023;17:79–86. doi: 10.21763/tjfmpc.1132131. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

