A Sensitive and Accurate Electrochemical Sensor Based on Biomass-Derived Porous Carbon for the Detection of Ascorbic Acid
- PMID: 40733246
- PMCID: PMC12298935
- DOI: 10.3390/molecules30142980
A Sensitive and Accurate Electrochemical Sensor Based on Biomass-Derived Porous Carbon for the Detection of Ascorbic Acid
Abstract
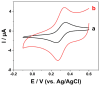
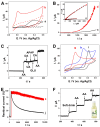
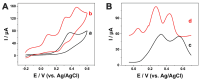
Ascorbic acid (AA) is a vital biomarker for human metabolic processes, and many diseases are strongly linked to aberrant variations in its content. It is crucial to detect the levels of AA with sensitivity, speed, and accuracy. In this work, three-dimensional honeycomb-like porous carbons derived from discarded walnut (green) husks (DWGH-HCPCs) were synthesized using a process involving hydrothermal treatment, freeze-drying, and carbonization. The DWGH-HCPCs, with a high specific surface area of 419.72 m2 g-1, large pore volume of 0.35 cm3 g-1 and high density of defective sites, are used to fabricate the electrochemical sensor for the detection of AA. The electrochemical performance of the DWGH-HCPC-modified glassy carbon electrode (GCE) (DWGH-HCPC/GCE) was investigated through chronoamperometry, differential pulse voltammetry, and cyclic voltammetry. Compared with the GCE, the DWGH-HCPC/GCE exhibits higher sensitivities (34.7 μA mM-1 and 22.7 μA mM-1), a wider linear range (10-1040 μM and 1040-3380 μM), and a lower detection limit (0.26 μM) for AA detection. Specifically, the real sample concentrations of AA in beverages and artificial urine were successfully identified by DWGH-HCPC/GCE. Additionally, the DWGH-HCPC/GCE demonstrated great feasibility in the simultaneous detection of AA, dopamine (DA), and uric acid (UA). Therefore, as a green, eco-friendly, and low-cost electrode modifier, DWGH-HCPCs have broad prospects in the development of electrochemical sensing platforms for food and medical applications.
Keywords: ascorbic acid; biomass; electrochemical sensor; porous carbon.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Morphine electrochemical determination using SnO2 nanostructure-modified glassy carbon electrode in the presence of diclofenac.ADMET DMPK. 2025 Jul 2;13(4):2803. doi: 10.5599/admet.2803. eCollection 2025. ADMET DMPK. 2025. PMID: 40786067 Free PMC article.
-
A new electrochemical sensor for the detection of uric acid and xanthine based on a carbon paste electrode coated with a metal organic framework.Anal Methods. 2025 Aug 7;17(31):6405-6419. doi: 10.1039/d5ay00832h. Anal Methods. 2025. PMID: 40735861
-
Design of molecularly imprinted polymer based electrochemical sensor for eco-friendly, selective, and sensitive determination of lincomycin in food samples.Food Chem. 2025 Oct 1;488:144883. doi: 10.1016/j.foodchem.2025.144883. Epub 2025 May 23. Food Chem. 2025. PMID: 40435642
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
A critical review of electrochemical and optical Vitamin B6 sensing: evolution of biosensor platforms based on advanced nanosystems.Crit Rev Food Sci Nutr. 2025;65(22):4243-4263. doi: 10.1080/10408398.2024.2386037. Epub 2024 Aug 8. Crit Rev Food Sci Nutr. 2025. PMID: 39115011 Review.
References
-
- Hussein A.Z.M. Spectrophotometric Determination of Ascorbic acid in Aqueous Solutions and in Pharmaceuticals formulations. Al-Nahrain J. Sci. 2013;16:65–71. doi: 10.22401/JNUS.16.3.08. - DOI
-
- Muslim Mohabis R., Fazeli F., Amini I., Azizkhani V. An overview of recent advances in the detection of ascorbic acid by electrochemical techniques. J. Electrochem. Sci. Eng. 2022;12:1081–1098. doi: 10.5599/jese.1561. - DOI
-
- Chen X., Yang Z., Tuo X., Huang H., Huang J., Li L., Yu X.J. Sea-urchin-structured NiCo2O4 decorated nitrogen-doped graphene for enhanced electrochemical detection of ascorbic acid. Solid State Sci. 2022;133:107000. doi: 10.1016/j.solidstatesciences.2022.107000. - DOI
-
- Qu C., Li H., Zhou S., Li G., Wang C., Snyders R., Bittencourt C., Li W. Bi2S3/rGO composite based electrochemical sensor for ascorbic acid detection. Chemosensors. 2021;9:190. doi: 10.3390/chemosensors9080190. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

