Reversible Binding of Nitric Oxide in a Cu(II)-Containing Microporous Metal-Organic Framework
- PMID: 40733273
- PMCID: PMC12298806
- DOI: 10.3390/molecules30143007
Reversible Binding of Nitric Oxide in a Cu(II)-Containing Microporous Metal-Organic Framework
Abstract
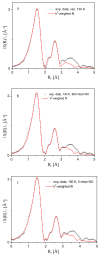
We studied the adsorption thermodynamics and mechanism behind the binding of nitric oxide (NO) in the interior surfaces and structural fragments of the high metal center density microporous Metal-Organic Framework (MOF) CPO-27-Cu, by gas sorption, at a series of temperatures. For the purpose of comparison, we also measured the corresponding CO2 adsorption isotherms, and as a result, the isosteric heats of adsorption for the two studied adsorptives were derived, being in the range of 12-15 kJ/mol for NO at loadings up to 0.5 NO molecules per formula unit (f.u.) of the bare compound (C4O3HCu), and 23-25 kJ/mol CO2 in the range 0-1 CO2 per f.u. Microscopically, the mode of NO binding near the square pyramid Cu(II) centers was directly accessed with the use of in situ NO gas adsorption X-ray Absorption Spectroscopy (XAS). Additionally, during the vacuum/temperature activation of the material and consequent NO adsorption, the electronic state of the Cu-species was monitored by observing the corresponding X-ray Near Edge Spectra (XANES). Contrary to the previously anticipated chemisorption mechanism for NO binding at Cu(II) species, we found that at slightly elevated temperatures, under ambient, but also cryogenic conditions, only relatively weak physisorption takes place, with no evidence for a particular adsorption preference to the coordinatively unsaturated Cu-centers of the material.
Keywords: EXAFS; adsorption; gas storage; microporosity; nitric oxide; thermodynamics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Halogen-Decorated Metal-Organic Frameworks for Efficient and Selective CO2 Capture, Separation, and Chemical Fixation with Epoxides under Mild Conditions.ACS Appl Mater Interfaces. 2024 Apr 24;16(16):20626-20641. doi: 10.1021/acsami.4c02560. Epub 2024 Apr 11. ACS Appl Mater Interfaces. 2024. PMID: 38605636
-
Active body surface warming systems for preventing complications caused by inadvertent perioperative hypothermia in adults.Cochrane Database Syst Rev. 2016 Apr 21;4(4):CD009016. doi: 10.1002/14651858.CD009016.pub2. Cochrane Database Syst Rev. 2016. PMID: 27098439 Free PMC article.
-
Enhanced and efficient capture of Cd(II) through functionalized metal-organic frameworks embedded in a biopolymer (carboxymethyl cellulose/polyethylenimine): Thermodynamics, kinetics, and optimization via Box-Behnken methodology.Int J Biol Macromol. 2025 Jul;318(Pt 1):144903. doi: 10.1016/j.ijbiomac.2025.144903. Epub 2025 Jun 4. Int J Biol Macromol. 2025. PMID: 40473180
-
Inhaled nitric oxide for treating pain crises in people with sickle cell disease.Cochrane Database Syst Rev. 2022 Jul 8;7(7):CD011808. doi: 10.1002/14651858.CD011808.pub3. Cochrane Database Syst Rev. 2022. PMID: 35802341 Free PMC article.
-
Transcutaneous electrical nerve stimulation (TENS) for fibromyalgia in adults.Cochrane Database Syst Rev. 2017 Oct 9;10(10):CD012172. doi: 10.1002/14651858.CD012172.pub2. Cochrane Database Syst Rev. 2017. PMID: 28990665 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

