Impact of Plant Developmental Stage on Photosynthetic Acclimation to Elevated [CO2] in Durum Wheat
- PMID: 40733461
- PMCID: PMC12299461
- DOI: 10.3390/plants14142224
Impact of Plant Developmental Stage on Photosynthetic Acclimation to Elevated [CO2] in Durum Wheat
Abstract
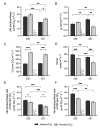
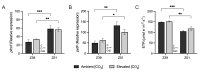
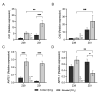
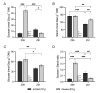
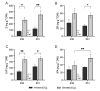
The response of plants to elevated atmospheric [CO2] is highly dynamic and influenced by developmental stage, yet its role in photosynthetic acclimation remains underexplored. This study examines the physiological and molecular responses of wheat (Triticum durum, var. Amilcar) to elevated [CO2] (700 ppm vs. 400 ppm) at two distinct developmental stages: the vegetative stage at the end of the elongation stage and the reproductive stage at the beginning of ear emergence (Z39 and Z51, respectively). Wheat plants at the developmental stage Z39, cultivated under elevated [CO2], maintained photosynthetic rates despite a carbohydrate build-up. However, at Z51, photosynthetic acclimation became more evident as the decline in Rubisco carboxylation capacity (Vcmax) persisted, but also stomatal conductance and diffusion were decreased. This was accompanied by the up-regulation of the CA1 and CA2 genes, likely as a compensatory mechanism to maintain CO2 supply. Additionally, hormonal adjustments under elevated [CO2], including increased auxin and bioactive cytokinins (zeatin and isopentenyl adenine), may have contributed to delayed senescence and nitrogen remobilization, sustaining carbon assimilation despite biochemical constraints. These findings highlight the developmental regulation of photosynthetic acclimation, emphasizing the need for the stage-specific assessments of crop responses to future atmospheric conditions.
Keywords: carbohydrates; elevated [CO2]; nitrogen; photosynthetic acclimation; phytohormones.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial, financial or personal relationships that could be construed as a potential conflict of interest.
Figures
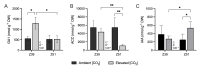
References
-
- IPCC . In: Climate Change 2022: Impacts, Adaptation and Vulnerability. Pörtner H.-O., Roberts D.C., Tignor M., Poloczanska E.S., Mintenbeck K., Alegría A., Craig M., Langsdorf S., Löschke S., Möller V., et al., editors. Cambridge University Press; Cambridge, UK: New York, NY, USA: 2022. 3056p. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. - DOI
-
- Ainsworth E.A., Rogers A., Nelson R., Long S.P. Testing the “Source-Sink” Hypothesis of down-Regulation of Photosynthesis in Elevated [CO2] in the Field with Single Gene Substitutions in Glycine Max. Agric. For. Meteorol. 2004;122:85–94. doi: 10.1016/j.agrformet.2003.09.002. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

