Integrated In Silico, In Vitro, and In Vivo Studies Reveal Mangiferin as a Promising Antiviral Agent Against H1N1/pdm2009 Influenza Virus
- PMID: 40733492
- PMCID: PMC12299334
- DOI: 10.3390/v17070873
Integrated In Silico, In Vitro, and In Vivo Studies Reveal Mangiferin as a Promising Antiviral Agent Against H1N1/pdm2009 Influenza Virus
Abstract
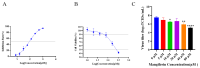
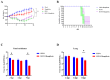
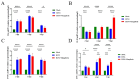
The ongoing global threat posed by the influenza A virus, exacerbated by antigenic drift and the emergence of antiviral resistance, accentuates the urgent need for innovative therapeutic strategies. Through molecular docking, this study revealed that mangiferin has a strong binding affinity for the active site of the neuraminidase (NA) protein of influenza virus A(H1N1)pdm09, with a binding energy of -8.1 kcal/mol. In vitro assays confirmed a dose-dependent inhibition of NA, with an IC50 of 88.65 μM, and minimal cytotoxicity, as indicated by a CC50 of 328.1 μM in MDCK cells. In murine models, the administration of mangiferin at a dosage of 25 mg/kg significantly mitigated weight loss, decreased viral loads in nasal turbinates and lungs by over 1 log10 TCID50, and enhanced survival rates from 0% in control groups to 20% in mangiferin-treated group at 14 days post-infection. In addition, mangiferin was found to modulate host immune responses by simultaneously inhibiting pro-inflammatory cytokines, IL-6 and TNF-α, and upregulating the expression of anti-inflammatory IL-10 and antiviral IFN-γ, thus mitigating infection-induced inflammation. Our findings elucidate the dual mechanism of mangiferin involving the direct inhibition of NA and immunomodulation, thereby providing experimental evidence for exploring dual-mechanism-based anti-influenza strategies against resistant strains of influenza.
Keywords: influenza virus A(H1N1)pdm09; mangiferin; molecular docking.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
The mechanism of action in Mussaenda pubescens (Yuye Jinhua) against influenza A virus: Evidence from in vitro and in vivo studies.Phytomedicine. 2025 Sep;145:157070. doi: 10.1016/j.phymed.2025.157070. Epub 2025 Jul 13. Phytomedicine. 2025. PMID: 40684492
-
Synergistic Antiviral Activity of Xanthan Gum and Camostat Against Influenza Virus Infection.Viruses. 2025 Feb 21;17(3):301. doi: 10.3390/v17030301. Viruses. 2025. PMID: 40143232 Free PMC article.
-
Protein Disulfide Isomerase A3 Regulates Influenza Neuraminidase Activity and Influenza Burden in the Lung.Int J Mol Sci. 2022 Jan 19;23(3):1078. doi: 10.3390/ijms23031078. Int J Mol Sci. 2022. PMID: 35162999 Free PMC article.
-
Systematic review of influenza resistance to the neuraminidase inhibitors.BMC Infect Dis. 2011 May 19;11:134. doi: 10.1186/1471-2334-11-134. BMC Infect Dis. 2011. PMID: 21592407 Free PMC article.
-
Neuraminidase inhibitors for preventing and treating influenza in adults and children.Cochrane Database Syst Rev. 2014 Apr 10;2014(4):CD008965. doi: 10.1002/14651858.CD008965.pub4. Cochrane Database Syst Rev. 2014. PMID: 24718923 Free PMC article.
References
-
- Emborg H., Botnen A.B., Nielsen J., Vestergaard L.S., Lomholt F.K., Munkstrup C., Moller K.L., Kjelso C., Rasmussen S.H., Trebbien R. Age-dependent influenza infection patterns and subtype circulation in Denmark, in seasons 2015/16 to 2021/22. Eurosurveillance. 2024;29:2300263. doi: 10.2807/1560-7917.ES.2024.29.4.2300263. - DOI - PMC - PubMed
-
- van der Vries E., Collins P.J., Vachieri S.G., Xiong X., Liu J., Walker P.A., Haire L.F., Hay A.J., Schutten M., Osterhaus A.D.M.E., et al. H1N1 2009 pandemic influenza virus: Resistance of the I223R neuraminidase mutant explained by kinetic and structural analysis. PLoS Pathog. 2012;8:e1002914. doi: 10.1371/journal.ppat.1002914. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

