Interactions Between Hantavirus Nucleoprotein and Glycoproteins: A Quantitative Fluorescence Microscopy Study
- PMID: 40733557
- PMCID: PMC12298772
- DOI: 10.3390/v17070940
Interactions Between Hantavirus Nucleoprotein and Glycoproteins: A Quantitative Fluorescence Microscopy Study
Abstract
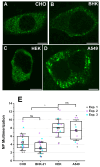
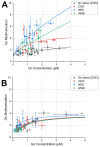
Orthohantaviruses are tri-segmented negative-sense RNA viruses that can cause severe pathologies in humans. Currently, limited information exists on the molecular interactions driving orthohantavirus assembly in infected cells. Specifically, it is not clear how its glycoproteins (i.e., Gn and Gc) interact with other viral or host molecules. In this study, we use one- and two-color Number and Brightness fluorescence microscopy approaches to quantitatively characterize the interactions between orthohantavirus glycoproteins and the nucleoprotein in transfected cells. Our results indicate that orthohantavirus nucleoprotein homo-interactions are strongly affected by the host environment. Furthermore, we report evidence of Gc-nucleoprotein interactions, based on (i) the high fluorescence cross-correlation between these two proteins and (ii) the increased Gc-Gc interactions observed in the presence of nucleoprotein. Finally, experiments on a Gc deletion mutant suggest that the observed protein-protein interactions are mediated by the cytoplasmic tail of Gc. In conclusion, this study provides new insights into the role of the interactions between orthohantavirus glycoproteins and nucleoprotein in the context of viral assembly.
Keywords: fluorescence fluctuations; fluorescence microscopy; fluorescent proteins; multimerization; protein–protein interactions; virus assembly.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Glycoproteins gM and gN are indispensable factors for rhesus macaque rhadinovirus replication and spread but can be reconstituted by KSHV chimeras.J Virol. 2025 Mar 18;99(3):e0192224. doi: 10.1128/jvi.01922-24. Epub 2025 Feb 25. J Virol. 2025. PMID: 39998253 Free PMC article.
-
Viral polymerase-host interaction analysis reveals that the association between avian NUP93 and PB1 promotes H5N6 avian influenza virus replication.Microbiol Spectr. 2025 Jul;13(7):e0312024. doi: 10.1128/spectrum.03120-24. Epub 2025 May 23. Microbiol Spectr. 2025. PMID: 40407330 Free PMC article.
-
EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update.Eur J Cancer. 2007 Jan;43(2):258-70. doi: 10.1016/j.ejca.2006.10.014. Epub 2006 Dec 19. Eur J Cancer. 2007. PMID: 17182241
-
123I-MIBG scintigraphy and 18F-FDG-PET imaging for diagnosing neuroblastoma.Cochrane Database Syst Rev. 2015 Sep 29;2015(9):CD009263. doi: 10.1002/14651858.CD009263.pub2. Cochrane Database Syst Rev. 2015. PMID: 26417712 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

