Homoharringtonine Inhibits CVS-11 and Clinical Isolates of Rabies Virus In Vitro: Identified via High-Throughput Screening of an FDA-Approved Drug Library
- PMID: 40733562
- PMCID: PMC12299688
- DOI: 10.3390/v17070945
Homoharringtonine Inhibits CVS-11 and Clinical Isolates of Rabies Virus In Vitro: Identified via High-Throughput Screening of an FDA-Approved Drug Library
Abstract
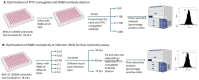
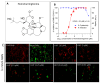
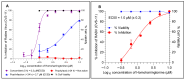
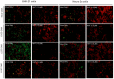
Rabies, a viral encephalitis caused by rabies virus (RABV), is 100% fatal upon the onset of symptoms. Effective post-exposure prophylaxis (PEP) measures are available, but they are often difficult to access in low-income countries. WHO estimates about 59,000 deaths due to rabies globally, and the majority are contributed by developing countries. Hence, developing drugs for the treatment of post-symptomatic rabies is an urgent and unmet demand. It is worth noting that previous efforts regarding antiviral strategies, such as small-interfering RNA, antibodies and small-molecule inhibitors, against the rabies virus have failed to show efficacy in pre-clinical studies, especially when the virus has reached the central nervous system (CNS). Therefore, drug repurposing seems to be an alternative tool for the development of new anti-rabies drugs. We validated and used a high-throughput, FITC-conjugated antibody-based flow cytometry assay to expedite the identification of repurposable new drug candidates against the RABV. The assay was validated using ribavirin and salinomycin as reference compounds, which showed EC50 values of 10.08 µM and 0.07 µM, respectively. We screened a SelleckChem library comprising 3035 FDA-approved compounds against RABV (CVS-11) at 10 µM concentration. Five compounds (clofazimine, tiamulin, difloxacin, harringtonine and homoharringtonine) were active against RABV, with greater than 90% inhibition. Homoharringtonine (HHT) identified in the present study is active against laboratory-adapted RABV (CVS-11) and clinical isolates of RABV, with an average EC50 of 0.3 µM in both BHK-21 and Neuro-2a cell lines and exhibits post-entry inhibition.
Keywords: CVS-11; RABV; clinical isolate; flow cytometry; high-throughput assay; homoharringtonine.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Cantaert T., Borand L., Kergoat L., Leng C., Ung S., In S., Peng Y., Phoeun C., Hing C., Taing C.N., et al. A 1-Week Intradermal Dose-Sparing Regimen for Rabies Post-Exposure Prophylaxis (RESIST-2): An Observational Cohort Study. Lancet Infect. Dis. 2019;19:1355–1362. doi: 10.1016/S1473-3099(19)30311-1. - DOI - PubMed
-
- World Health Organization . WHO Expert Consultation on Rabies: Third Report. World Health Organization; Geneva, Switzerland: 2018.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

