Spatiotemporal Characterization of Changes in the Respiratory Tract and the Nervous System, Including the Eyes in SARS-CoV-2-Infected K18-hACE2 Mice
- PMID: 40733579
- PMCID: PMC12300873
- DOI: 10.3390/v17070963
Spatiotemporal Characterization of Changes in the Respiratory Tract and the Nervous System, Including the Eyes in SARS-CoV-2-Infected K18-hACE2 Mice
Abstract
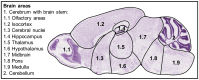
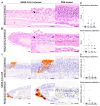
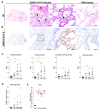
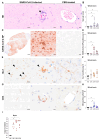
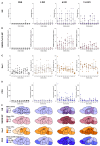
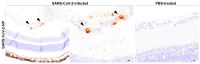
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), is known to affect multiple organ systems, including the respiratory tract and nervous and ocular systems. This retrospective study aimed to characterize the spatiotemporal distribution of viral antigen and associated pathological changes in the nose, lungs, brain, and eyes of K18-hACE2 mice intranasally infected with SARS-CoV-2. Using histology and immunohistochemistry, tissues were examined at 3, 6, and 7/8 days post-infection (dpi). In addition, lung and brain tissues were analyzed by means of RT-qPCR to determine viral RNA titers. Viral antigen was most pronounced in the nose, brain, and lung at 3, 6, and 7/8 dpi, respectively, whereas viral antigen was detected at 6 and 7/8 dpi in the retina. Quantitative PCR confirmed increasing viral RNA levels in both lung and brain, peaking at 7/8 dpi. Nasal and lung inflammation mirrored viral antigen distribution and localization. In the brain, the predominantly basal viral spread correlated with lymphohistiocytic meningoencephalitis, neuronal vacuolation, and altered neurofilament immunoreactivity. Retinal ganglion cells showed viral antigen expression without associated lesions. Microglial activation was evident in both the optic chiasm and the brain. These findings highlight the K18-hACE2 model's utility for studying extrapulmonary SARS-CoV-2 pathogenesis. Understanding the temporal and spatial dynamics of viral spread enhances insights into SARS-CoV-2 neurotropism and its clinical manifestations.
Keywords: BavPat1; CNS; COVID-19; K18-hACE2 mice; SARS-CoV-2; central nervous system; neuropathology; ocular manifestation; severe acute respiratory syndrome coronavirus 2; spatiotemporal distribution; upper and lower respiratory tract.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Severe Acute Respiratory Syndrome Coronavirus 2 Variant Infection Dynamics and Pathogenesis in Transgenic K18-hACE2 and Inbred Immunocompetent C57BL/6J Mice.Viruses. 2025 Mar 30;17(4):500. doi: 10.3390/v17040500. Viruses. 2025. PMID: 40284943 Free PMC article.
-
Establishment and characterization of an hACE2/hTMPRSS2 knock-in mouse model to study SARS-CoV-2.Front Immunol. 2024 Jul 10;15:1428711. doi: 10.3389/fimmu.2024.1428711. eCollection 2024. Front Immunol. 2024. PMID: 39050847 Free PMC article.
-
Neurobiological Alterations Induced by SARS-CoV-2: Insights from Variant-Specific Host Gene Expression Patterns in hACE2-Expressing Mice.Viruses. 2025 Feb 27;17(3):329. doi: 10.3390/v17030329. Viruses. 2025. PMID: 40143258 Free PMC article.
-
Laboratory-based molecular test alternatives to RT-PCR for the diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Oct 14;10(10):CD015618. doi: 10.1002/14651858.CD015618. Cochrane Database Syst Rev. 2024. PMID: 39400904
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article.
References
-
- Vashishtha V.M., Kumar P. Preliminary clinical characteristics of pediatric COVID-19 cases during the ongoing Omicron XBB.1.16 driven surge in a north Indian city. medRxiv. 2023:2023.2004.2018.23288715. doi: 10.1101/2023.04.18.23288715. - DOI
MeSH terms
Substances
Grants and funding
- 14 - 76103-184 CORONA-15/20/Ministry of Science and Culture of Lower Saxony, Germany
- 398066876/GRK 2485/2/Deutsche Forschungsgemeinschaft (DFG; German Research Foundation)
- AFR15686728/National Research Fund (FNR), Luxembourg
- not applicable/University of Veterinary Medicine, Foundation, Hannover, Germany
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

