The Cytoplasmic Tail of Ovine Herpesvirus 2 Glycoprotein B Affects Cell Surface Expression and Is Required for Membrane Fusion
- PMID: 40733610
- PMCID: PMC12298039
- DOI: 10.3390/v17070994
The Cytoplasmic Tail of Ovine Herpesvirus 2 Glycoprotein B Affects Cell Surface Expression and Is Required for Membrane Fusion
Abstract
Ovine herpesvirus 2 (OvHV-2) causes the fatal veterinary disease malignant catarrhal fever (MCF). Fusion is an essential step in the host cell entry of enveloped viruses and is an important target for vaccine development. OvHV-2 cannot be propagated in vitro, so a robust virus-free cell-cell membrane fusion assay is necessary to elucidate its entry mechanism. OvHV-2 cell-cell fusion requires three conserved herpesviral envelope glycoproteins: gB, gH, and gL. OvHV-2 fusion activity is detectable but low. We hypothesize that enhancing the cell surface expression of gB, which is the core herpesviral fusogen, will increase cell-cell fusion. We generated C-terminal truncation mutants of gB and determined their cell surface expression, subcellular distribution, and fusion activity. Two mutants, including one that lacked the entire cytoplasmic tail domain, failed to function in the cell-cell fusion assay, despite wild-type levels of surface expression. This suggests that the OvHV-2 gB cytoplasmic tail is critical for fusion. A gB mutant truncated at amino acid 847 showed increased surface expression and fusion relative to the wild type. This suggests that the robust fusion activity of gB847 is the result of increased surface expression. gB847 may be used in place of wild-type gB in an improved, more robust OvHV-2 fusion assay.
Keywords: fusion; glycoprotein B; ovine herpesvirus 2; surface expression.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; the collection, analysis, or interpretation of data; the writing of the manuscript; or the decision to publish the results.
Figures

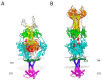



Similar articles
-
Cell Fusion Induced by a Fusion-Active Form of Human Cytomegalovirus Glycoprotein B (gB) Is Inhibited by Antibodies Directed at Antigenic Domain 5 in the Ectodomain of gB.J Virol. 2020 Aug 31;94(18):e01276-20. doi: 10.1128/JVI.01276-20. Print 2020 Aug 31. J Virol. 2020. PMID: 32641474 Free PMC article.
-
Ovine Herpesvirus 2 Glycoproteins B, H, and L Are Sufficient for, and Viral Glycoprotein Ov8 Can Enhance, Cell-Cell Membrane Fusion.J Virol. 2017 Feb 28;91(6):e02454-16. doi: 10.1128/JVI.02454-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28053110 Free PMC article.
-
Human cytomegalovirus gH/gL/gO binding to PDGFRα provides a regulatory signal activating the fusion protein gB that can be blocked by neutralizing antibodies.J Virol. 2025 May 20;99(5):e0003525. doi: 10.1128/jvi.00035-25. Epub 2025 Apr 9. J Virol. 2025. PMID: 40202318 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
References
-
- Nelson D.D., Davis W.C., Brown W.C., Li H., O’Toole D., Oaks J.L. CD8+/perforin+/WC1− γδ T cells, not CD8+ αβ T cells, infiltrate vasculitis lesions of American bison (Bison bison) with experimental sheep-associated malignant catarrhal fever. Vet. Immunol. Immunopathol. 2010;136:284–291. doi: 10.1016/j.vetimm.2010.03.023. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

