The Renin-Angiotensin System Modulates SARS-CoV-2 Entry via ACE2 Receptor
- PMID: 40733630
- PMCID: PMC12299366
- DOI: 10.3390/v17071014
The Renin-Angiotensin System Modulates SARS-CoV-2 Entry via ACE2 Receptor
Abstract
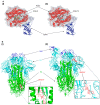
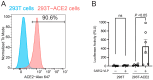
The renin-angiotensin system (RAS) plays a central role in cardiovascular regulation and has gained prominence in the pathogenesis of Coronavirus Disease 2019 (COVID-19) due to the critical function of angiotensin-converting enzyme 2 (ACE2) as the entry receptor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Angiotensin IV, but not angiotensin II, has recently been reported to enhance the binding between the viral spike protein and ACE2. To investigate the virological significance of this effect, we developed a single-round infection assay using SARS-CoV-2 viral-like particles expressing the spike protein. Our results demonstrate that while angiotensin II does not affect viral infectivity across concentrations ranging from 40 nM to 400 nM, angiotensin IV enhances viral entry at a low concentration but exhibits dose-dependent inhibition at higher concentrations. These findings highlight the unique dual role of angiotensin IV in modulating SARS-CoV-2 entry. In silico molecular docking simulations indicate that angiotensin IV was predicted to associate with the S1 domain near the receptor-binding domain in the open spike conformation. Given that reported plasma concentrations of angiotensin IV range widely from 17 pM to 81 nM, these levels may be sufficient to promote, rather than inhibit, SARS-CoV-2 infection. This study identifies a novel link between RAS-derived peptides and SARS-CoV-2 infectivity, offering new insights into COVID-19 pathophysiology and informing potential therapeutic strategies.
Keywords: angiotensin; angiotensin-converting enzyme 2; renin–angiotensin system; severe acute respiratory syndrome coronavirus 2; spike protein; viral-like particle.
Conflict of interest statement
No conflicts of interest.
Figures


Update of
-
The Renin-Angiotensin System Modulates SARS-CoV-2 Entry via ACE2 Receptor.bioRxiv [Preprint]. 2025 Jun 25:2025.06.25.661409. doi: 10.1101/2025.06.25.661409. bioRxiv. 2025. Update in: Viruses. 2025 Jul 19;17(7):1014. doi: 10.3390/v17071014. PMID: 40666850 Free PMC article. Updated. Preprint.
Similar articles
-
The Renin-Angiotensin System Modulates SARS-CoV-2 Entry via ACE2 Receptor.bioRxiv [Preprint]. 2025 Jun 25:2025.06.25.661409. doi: 10.1101/2025.06.25.661409. bioRxiv. 2025. Update in: Viruses. 2025 Jul 19;17(7):1014. doi: 10.3390/v17071014. PMID: 40666850 Free PMC article. Updated. Preprint.
-
Determinants of susceptibility to SARS-CoV-2 infection in murine ACE2.J Virol. 2025 Jun 17;99(6):e0054325. doi: 10.1128/jvi.00543-25. Epub 2025 May 12. J Virol. 2025. PMID: 40353671 Free PMC article.
-
Critical amino acid residues in human ACE2 for SARS-CoV-2 spike protein binding and virus entry.Microbiol Spectr. 2025 Aug 5;13(8):e0324424. doi: 10.1128/spectrum.03244-24. Epub 2025 Jun 20. Microbiol Spectr. 2025. PMID: 40539804 Free PMC article.
-
Receptor Binding for the Entry Mechanisms of SARS-CoV-2: Insights from the Original Strain and Emerging Variants.Viruses. 2025 May 10;17(5):691. doi: 10.3390/v17050691. Viruses. 2025. PMID: 40431702 Free PMC article. Review.
-
ACE2 (Angiotensin-Converting Enzyme 2) in Cardiopulmonary Diseases: Ramifications for the Control of SARS-CoV-2.Hypertension. 2020 Sep;76(3):651-661. doi: 10.1161/HYPERTENSIONAHA.120.15595. Epub 2020 Aug 12. Hypertension. 2020. PMID: 32783758 Free PMC article. Review.
References
-
- Zhang P., Yu L., Dong J., Liu Y., Zhang L., Liang P., Wang L., Chen B., Huang L., Song C. Cellular poly(C) binding protein 2 interacts with porcine epidemic diarrhea virus papain-like protease 1 and supports viral replication. Vet. Microbiol. 2020;247:108793. doi: 10.1016/j.vetmic.2020.108793. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

