Factors Associated with Impaired Humoral Immune Response to mRNA Vaccines in Patients with Inflammatory Bowel Disease: A Matched-Cohort Analysis from the RisCoin Study
- PMID: 40733650
- PMCID: PMC12299133
- DOI: 10.3390/vaccines13070673
Factors Associated with Impaired Humoral Immune Response to mRNA Vaccines in Patients with Inflammatory Bowel Disease: A Matched-Cohort Analysis from the RisCoin Study
Abstract
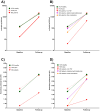
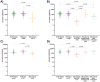
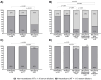
Background/Objectives: The SARS-CoV-2 pandemic challenged patients with inflammatory bowel disease (IBD) under immunosuppressive therapies. We used data from the RisCoin cohort to investigate factors associated with a poor immune response to mRNA vaccination in these patients. Methods: From 4115 RisCoin participants, we matched 110 IBD patients by age and time interval since the second mRNA vaccination with 306 healthcare workers (HCW) without comorbidities (HCW-healthy) and 292 with medical conditions (HCW-plus); all were SARS-CoV-2 infection naïve. Basic questionnaires collected data on medication, COVID-19 vaccinations and side-effects, dietary patterns, lifestyle factors, and self-perceived stress. Main outcomes included anti-spike immunoglobulin levels and antibody-mediated live-virus neutralization immunity (NT) to the Omicron BA.1 variant (threshold NT ≥ 10 defined as IC50 values ≥1:10 serum dilution) after the second (baseline) and third vaccinations. Results: At baseline, IBD patients treated with anti-TNF but not those under vedolizumab or ustekinumab therapy had lower anti-spike levels compared to HCW-healthy and HCW-plus (166 versus 1384 and 1258 BAU/mL, respectively; p < 0.0001). Anti-TNF compared to vedolizumab/ustekinumab-treated patients reached NT titers above threshold in 17% versus 64%, respectively, and HCW-subgroups in 73% and 79% (all p < 0.0001). Current smokers showed a four to five times increased risk for non-neutralizing immunity compared to non-smokers. After the third vaccination, NT titers did not reach threshold in 15% anti-TNF compared to 5% vedolizumab/ustekinumab-treated patients and none of HCW (p < 0.01). Patients with IBD reported fewer clinical symptoms after vaccination. Perceived stress was not increased. Conclusions: Our findings support individualized schedules for mRNA-based vaccines in IBD patients with different immunosuppressive therapies and enforcement of non-smoking.
Keywords: SARS-CoV-2; anti-spike antibodies; perceived stress questionnaire; virus neutralizing immunity.
Conflict of interest statement
L.K. reports consultant fees from Janssen-Cilag and Takeda and lecture honoraria from Falk Foundation outside the submitted work. TS received lecture honoraria from Nutricia and MSD, travel support from AbbVie and Ferring, and consulting fees from AstraZeneca outside the submitted work. H.P.T. reports consultant fees from AbbVie, Calypso Biotech, Immunic Janssen-Cilag, and Pharmacosmos and lecture honoraria from AbbVie, Biogen, BMS, Falk Foundation, Galapagos, Janssen-Cilag Pfizer, Pharmacosmos, and Takeda Pharma outside the submitted work. SK reports personal fees from AbbVie, AstraZeneca, Danone, Janssen, Mead Johnson, Nestle Nutrition, Pfizer, Sanofi, Takeda, and Tillotts outside the submitted work. All authors declare no conflicts of interest to this published work. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Comparison of SARS-CoV-2 IgG responses in hemodialysis patients and healthcare workers after COVID-19 vaccination.Front Immunol. 2025 Jul 15;16:1586468. doi: 10.3389/fimmu.2025.1586468. eCollection 2025. Front Immunol. 2025. PMID: 40735320 Free PMC article.
-
COVID-19 Vaccines.2025 Jul 15. Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006–. 2025 Jul 15. Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006–. PMID: 33355732 Free Books & Documents. Review.
References
-
- Koletzko L., Klucker E., Le Thi T.G., Breiteneicher S., Rubio-Acero R., Neuhaus L., Stark R.G., Standl M., Wieser A., Török H. Following pediatric and adult IBD patients through the COVID-19 pandemic: Changes in psychosocial burden and perception of infection risk and harm over time. J. Clin. Med. 2021;10:4124. doi: 10.3390/jcm10184124. - DOI - PMC - PubMed
-
- Schmidt C., Stallmach A., Sturm A., Bachmann O., Helwig U., Koletzko S., Lynen P., Schnoy E., Dignass A., Kucharzik T. Update: Addendum to S3-Guidelines Crohn disease and ulcerative colitis: Management of Patients with Inflammatory Bowel Disease with regard to COVID-19 (version 2.0) Z. Gastroenterol. 2024;62:517–534. - PubMed
-
- Long M.D., Parlett L., Lewis J.D., Haynes K., Adimadhyam S., Hou L., Wolfe A., Toh S., Burris J., Dorand J., et al. Corticosteroids but not Anti-TNF Are Associated With Increased COVID-19 Complications in Patients With Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2024;30:1345–1352. doi: 10.1093/ibd/izad176. - DOI - PubMed
-
- Brenner E.J., Ungaro R.C., Gearry R.B., Kaplan G.G., Kissous-Hunt M., Lewis J.D., Ng S.C., Rahier J.-F., Reinisch W., Ruemmele F.M. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: Results from an international registry. Gastroenterology. 2020;159:481–491.e483. doi: 10.1053/j.gastro.2020.05.032. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

