The Application of Single-Cell Technologies for Vaccine Development Against Viral Infections
- PMID: 40733664
- PMCID: PMC12300521
- DOI: 10.3390/vaccines13070687
The Application of Single-Cell Technologies for Vaccine Development Against Viral Infections
Abstract
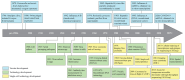
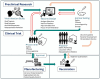
The development of vaccines against viral infections has advanced rapidly over the past century, propelled by innovations in laboratory and molecular technologies. These advances have expanded the range of vaccine platforms beyond live-attenuated and inactivated vaccines to include recombinant platforms, such as subunit proteins and virus-like particles (VLPs), and more recently, mRNA-based vaccines, while also enhancing methods for evaluating vaccine performance. Despite these innovations, a persistent challenge remains: the inherent complexity and heterogeneity of immune responses continue to impede efforts to achieve consistently effective and durable protection across diverse populations. Single-cell technologies have emerged as transformative tools for dissecting this immune heterogeneity, providing comprehensive and granular insights into cellular phenotypes, functional states, and dynamic host-pathogen interactions. In this review, we examine how single-cell epigenomic, transcriptomic, proteomic, and multi-omics approaches are being integrated across all stages of vaccine development-from infection-informed discovery to guide vaccine design, to high-resolution evaluation of efficacy, and refinement of cell lines for manufacturing. Through representative studies, we highlight how insights from these technologies contribute to the rational design of more effective vaccines and support the development of personalized vaccination strategies.
Keywords: COVID-19; infectious diseases; influenza; mRNA; multi-omics; recombinant protein; single-cell technology; vaccine development; viral diseases; virus-like particles (VLPs).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Vaccines and Immunization. [(accessed on 26 March 2025)]. Available online: https://www.who.int/health-topics/vaccines-and-immunization#tab=tab_1.
-
- Health and Economic Benefits of Routine Childhood Immunizations in the Era of the Vaccines for Children Program—United States, 1994–2023. [(accessed on 26 March 2025)]; Available online: https://www.cdc.gov/mmwr/volumes/73/wr/mm7331a2.htm#:~:text=This%20calcu.... - PMC - PubMed
-
- A Brief History of Vaccines. [(accessed on 26 March 2025)]. Available online: https://www.who.int/news-room/spotlight/history-of-vaccination/a-brief-h....
-
- Global Polio Vaccination. [(accessed on 26 March 2025)]; Available online: https://www.cdc.gov/global-polio-vaccination/about/index.html.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

