Replication of Vectored Herpesvirus of Turkey (HVT) in a Continuous, Microcarrier-Independent Suspension Cell Line from Muscovy Duck
- PMID: 40733691
- PMCID: PMC12298404
- DOI: 10.3390/vaccines13070714
Replication of Vectored Herpesvirus of Turkey (HVT) in a Continuous, Microcarrier-Independent Suspension Cell Line from Muscovy Duck
Abstract
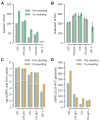
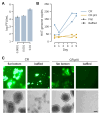
Background/Objectives: More than 33 billion chickens are industrially raised for meat and egg production globally and vaccinated against Marek's disease virus (MDV). The antigenically related herpesvirus of turkey (HVT) is used as a live-attenuated vaccine, commonly provided as a recombinant vector to protect chickens against additional unrelated pathogens. Because HVT replicates in a strictly cell-associated fashion to low levels of infectious units, adherent primary chicken or duck embryo fibroblasts are infected, dislodged from the cultivation surface and distributed as cryocultures in liquid nitrogen to the site of application. Although viable cells are complex products, application of infected cells in ovo confers protection even in presence of maternal antibodies. Methods/Results: The aim of our study was to determine whether a continuous cell line in a scalable cultivation format can be used for production of HVT-based vaccines. The AGE1.CR cell line (from Muscovy duck) was found to be highly permissive in adherent cultures. Propagation in suspension, however, initially gave very low yields. The induction of cell-to-cell contacts in carrier-independent suspensions and a metabolic shock improved titers to levels suitable for vaccine production (>105 infectious units/mL after infection with multiplicity of 0.001). Conclusions: Production of HVT is challenging to scale to large volumes and the reliance on embryonated eggs from biosecure facilities is complex. We demonstrate that a cell-associated HVT vector can be propagated in a carrier-independent suspension culture of AGE1.CR cells in chemically defined medium. The fed-batch production is independent of primary cells and animal-derived material and can be scaled to large volumes.
Keywords: HVT; MDV; Marek’s disease vaccine; suspension cell culture.
Conflict of interest statement
Authors K.M., S.A., D.H., A.K., V.S. and I.J. are employed by a company (ProBioGen AG) involved in vaccine research and development.
Figures




Similar articles
-
The Requirement of Turkey Herpesvirus (HVT) Glycoprotein C During Natural Infection in Chickens and Turkeys.Pathogens. 2025 May 28;14(6):538. doi: 10.3390/pathogens14060538. Pathogens. 2025. PMID: 40559546 Free PMC article.
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
Marek's disease virus replication in chicken skin reconstructed in vitro: evidence for viral particles in corneocytes.J Gen Virol. 2025 Jul;106(7):002123. doi: 10.1099/jgv.0.002123. J Gen Virol. 2025. PMID: 40694389 Free PMC article.
-
The Meq Genes of Nigerian Marek's Disease Virus (MDV) Field Isolates Contain Mutations Common to Both European and US High Virulence Strains.Viruses. 2024 Dec 31;17(1):56. doi: 10.3390/v17010056. Viruses. 2024. PMID: 39861844 Free PMC article.
-
Vaccines for preventing influenza in healthy children.Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD004879. doi: 10.1002/14651858.CD004879.pub5. Cochrane Database Syst Rev. 2018. PMID: 29388195 Free PMC article.
References
-
- Palya V., Tatár-Kis T., Mató T., Felföldi B., Kovács E., Gardin Y. Onset and Long-Term Duration of Immunity Provided by a Single Vaccination with a Turkey Herpesvirus Vector ND Vaccine in Commercial Layers. Vet. Immunol. Immunopathol. 2014;158:105–115. doi: 10.1016/j.vetimm.2013.11.008. - DOI - PubMed
-
- Fiddaman S.R., Dimopoulos E.A., Lebrasseur O., du Plessis L., Vrancken B., Charlton S., Haruda A.F., Tabbada K., Flammer P.G., Dascalu S., et al. Ancient Chicken Remains Reveal the Origins of Virulence in Marek’s Disease Virus. Science. 2023;382:1276–1281. doi: 10.1126/science.adg2238. - DOI - PubMed
LinkOut - more resources
Full Text Sources

