Virus-like Particles Produced in the Baculovirus System Protect Hares from European Brown Hare Syndrome Virus (EBHSV) Infection
- PMID: 40733708
- PMCID: PMC12299225
- DOI: 10.3390/vaccines13070731
Virus-like Particles Produced in the Baculovirus System Protect Hares from European Brown Hare Syndrome Virus (EBHSV) Infection
Abstract
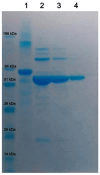
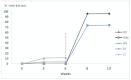
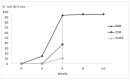
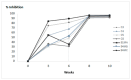
Background/Objectives: European Brown Hare Syndrome (EBHS) is an acute and highly contagious viral disease of hares that causes considerable economic losses on wild and captive-reared hares. No preventive treatments are currently available to defeat the disease. Immunoprophylactic and biosafety measures could be applied to prevent EBHS only in captive-reared hares, where vaccination is proposed as an effective strategy. Due to the lack of a cellular substrate for virus growth, commercially available vaccines are autovaccines produced from inactivated liver suspensions of hares dead for EBHS. Therefore, using a recombinant vaccine based on VP60 major capsid protein seems a viable alternative to overcome such a problem. Methods: the 6xHis C-terminal tagged VP60 protein of EBHSV was expressed and produced in baculovirus, purified by affinity chromatography and the self-assembled recombinant (rEVP60-His6) protein. To establish the protective properties of rEVP60-His6-based VLPs, hares were immunised with 50 and 100 µg of VLPs and parenterally challenged with EBHSV. Results: all hares vaccinated with 100 µg of VLPs survived after the experimental infection, demonstrating the excellent protective ability of this prototype VLPs-based vaccine. Conclusions: self-assembled EBHSV rEVP60-His6 protein was successfully produced following a rapid, simple, low-cost protocol. Although the protective efficacy of such VLPs were experimentally demonstrated, some key aspects remain to be clarified, including the duration of protection, the entity of the antibody response, and the ability to stimulate cell-mediated response. Last, an additional aspect to be evaluated is whether the use of an adjuvant can determine whether its presence improves the performance of the recombinant VLPs vaccine.
Keywords: EBHSV; European brown hare syndrome; VP60; vaccine; virus-like particles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Frölich K., Lavazza A. In: European Brown Hare Syndrome in Lagomorph Biology. Alves P.C., Ferrand N., Hacklaender K., editors. Springer; Berlin/Heidelberg, Germany: 2007. pp. 253–262.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

