Retinal Vascular Occlusion Following COVID-19 Vaccination: A Comprehensive Review of Observational Study and Pathophysiological Mechanisms
- PMID: 40733710
- PMCID: PMC12299647
- DOI: 10.3390/vaccines13070733
Retinal Vascular Occlusion Following COVID-19 Vaccination: A Comprehensive Review of Observational Study and Pathophysiological Mechanisms
Abstract
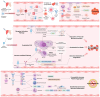
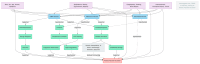
Background: Retinal vascular occlusion (RVO) and retinal artery occlusion (RAO) have been reported as rare adverse events following COVID-19 vaccination, raising concerns about vaccine safety. This review synthesizes cohort and case-control studies assessing the association between COVID-19 vaccines and RVO/RAO, while exploring potential pathophysiological mechanisms. Methods: We analyzed large-scale population-based studies from South Korea, Europe, and the TriNetX database, focusing on odds ratios (OR), hazard ratios (HR), and relative risks (RR) across mRNA and adenoviral vector vaccines. Pathological processes were hypothesized based on molecular and clinical evidence. Results: Studies investigating the association between COVID-19 vaccination and retinal vascular occlusion show conflicting results; some studies report no association (e.g., OR 0.93, 95% CI 0.60-1.45), others suggest reduced risk (e.g., OR 0.80, 95% CI 0.64-0.99), and one indicates increased risk over two years (HR 2.19, 95% CI 2.00-2.39). Adenoviral vector vaccines, particularly ChAdOx1, show higher RAO incidence in specific cohorts. Proposed mechanisms include vaccine-induced immune thrombotic thrombocytopenia (VITT) via anti-PF4 antibodies, spike protein-mediated endothelial dysfunction, and adjuvant-driven inflammation. Conclusions: While causality remains unproven, temporal heterogeneity and vaccine type-specific risks warrant further investigation. Longitudinal studies with robust controls are needed to clarify these associations in the post-pandemic context.
Keywords: COVID-19 vaccination; PF4 antibodies; adenoviral vector vaccines; mRNA vaccines; pathophysiological mechanisms; retinal artery occlusion (RAO); retinal vascular occlusion (RVO); spike protein; vaccine-induced immune thrombotic thrombocytopenia (VITT).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Vaccines for preventing infections in adults with haematological malignancies.Cochrane Database Syst Rev. 2025 May 21;5(5):CD015530. doi: 10.1002/14651858.CD015530.pub2. Cochrane Database Syst Rev. 2025. PMID: 40396505 Review.
-
Vaccines for preventing influenza in healthy children.Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD004879. doi: 10.1002/14651858.CD004879.pub5. Cochrane Database Syst Rev. 2018. PMID: 29388195 Free PMC article.
-
Efficacy and safety of COVID-19 vaccines.Cochrane Database Syst Rev. 2022 Dec 7;12(12):CD015477. doi: 10.1002/14651858.CD015477. Cochrane Database Syst Rev. 2022. PMID: 36473651 Free PMC article.
-
COVID-19 Vaccines.2025 Aug 15. Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006–. 2025 Aug 15. Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006–. PMID: 33355732 Free Books & Documents. Review.
-
Vaccines for preventing influenza in healthy adults.Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD001269. doi: 10.1002/14651858.CD001269.pub6. Cochrane Database Syst Rev. 2018. PMID: 29388196 Free PMC article.
References
-
- Badawi A.E., Elsheikh S.S., Addeen S.Z., Soliman M.A., Abd-Rabu R., Abdella W.S., Gad E.A. An Ophthalmic Insight into Novel Coronavirus 2019 Disease: A Comprehensive Review of the Ocular Manifestations and Clinical Hazards. J. Curr. Ophthalmol. 2020;32:315–328. doi: 10.4103/JOCO.JOCO_255_20. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

