Engaging Broader Stakeholders to Accelerate Group A Streptococcus Vaccine Development
- PMID: 40733711
- PMCID: PMC12299777
- DOI: 10.3390/vaccines13070734
Engaging Broader Stakeholders to Accelerate Group A Streptococcus Vaccine Development
Abstract
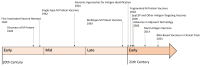
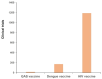
Group A Streptococcus (GAS) imposes a significant global health burden across all age groups, annually causing over 600 million cases of pharyngitis and more than 18 million severe invasive infections or sequelae. The resurgence of scarlet fever globally and streptococcal toxic shock syndrome (STSS) outbreaks in Japan have brought GAS infections back into the spotlight as a pressing global health concern. Unfortunately, no licensed vaccine against GAS is yet available for clinical use. Our comprehensive review examines the developmental history of GAS vaccines, outlining the research trajectory from early inactivated vaccines to contemporary multivalent, conjugate, multi-antigen, and mRNA-based vaccine platforms. It systematically analyzes clinical trial outcomes of GAS vaccines, highlighting recent advances in both M protein-based and non-M protein vaccine candidates while summarizing promising target antigens. The review concludes with critical strategies to accelerate vaccine commercialization, including enhanced investment in research and development, expanded collaborations, leveraging advanced vaccine technologies, streamlined clinical trials, and strengthened public health advocacy. This review critically evaluates the current evidence and future prospects in GAS vaccine development, emphasizing innovative strategies and engaging broader stakeholders to accelerate GAS vaccine development.
Keywords: GAS; Group A Streptococcus; J8; M protein; multivalent; vaccine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
Exploration of virulence and immune evasion functions of the candidate vaccine antigen SpyAD in the globally disseminated M1T1 group A Streptococcus strain.mBio. 2025 Jul 9;16(7):e0068325. doi: 10.1128/mbio.00683-25. Epub 2025 Jun 11. mBio. 2025. PMID: 40497733 Free PMC article.
-
Vaccines for preventing typhoid fever.Cochrane Database Syst Rev. 2018 May 31;5(5):CD001261. doi: 10.1002/14651858.CD001261.pub4. Cochrane Database Syst Rev. 2018. PMID: 29851031 Free PMC article.
-
Vaccines for preventing infections in adults with haematological malignancies.Cochrane Database Syst Rev. 2025 May 21;5(5):CD015530. doi: 10.1002/14651858.CD015530.pub2. Cochrane Database Syst Rev. 2025. PMID: 40396505 Review.
-
Vaccines for preventing influenza in healthy children.Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD004879. doi: 10.1002/14651858.CD004879.pub5. Cochrane Database Syst Rev. 2018. PMID: 29388195 Free PMC article.
References
-
- Lamagni T., Guy R., Chand M., Henderson K.L., Chalker V., Lewis J., Saliba V., Elliot A.J., Smith G.E., Rushton S., et al. Resurgence of scarlet fever in England, 2014–2016: A population-based surveillance study. Lancet Infect. Dis. 2018;18:180–187. doi: 10.1016/S1473-3099(17)30693-X. - DOI - PubMed
-
- Keuleyan E., Todorov T., Donchev D., Kevorkyan A., Vazharova R., Kukov A., Todorov G., Georgieva B., Altankova I., Uzunova Y., et al. Characterization of Streptococcus pyogenes Strains from Tonsillopharyngitis and Scarlet Fever Resurgence, 2023-FIRST Detection of M1UK in Bulgaria. Microorganisms. 2025;13:179. doi: 10.3390/microorganisms13010179. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources