Development of an Effective Single-Dose PCV2/CSFV Bivalent Subunit Vaccine Against Classical Swine Fever Virus and Porcine Circovirus Type 2
- PMID: 40733713
- PMCID: PMC12299614
- DOI: 10.3390/vaccines13070736
Development of an Effective Single-Dose PCV2/CSFV Bivalent Subunit Vaccine Against Classical Swine Fever Virus and Porcine Circovirus Type 2
Abstract
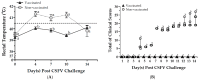
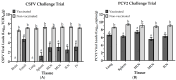
Background/Objectives: Porcine Circovirus Type 2 (PCV2) impairs pigs' immune systems and increases susceptibility to co-infections, including Classical Swine Fever (CSF), a highly contagious disease listed by the World Organisation for Animal Health (WOAH) as notifiable. Therefore, swine operations in CSF-endemic regions are encouraged to immunize piglets with both PCV2 and CSFV vaccinations. Currently, there is no commercially available bivalent vaccine for PCV2/CSFV. Methods: In this study, a total of twenty 4-week-old SPF pigs were administered our formulated PCV2/CSFV bivalent subunit vaccine, containing soluble CSFV-E2 (50 µg) and PCV2-ORF2 (100 µg) antigens with a porcine-specific CpG adjuvant. After 4 weeks of vaccination, all pigs were evaluated for efficacy against PCV2 and CSFV. Results: Pigs were only immunized once and showed significantly increased neutralizing or ELISA antibody titers against both viruses four weeks post-vaccination. After viral challenges, vaccinated pigs displayed no clinical signs or lesions and had markedly reduced CSFV and PCV2 viral loads in the serum and tissues compared to controls. Conclusions: These results demonstrate that a single dose of the PCV2/CSFV bivalent subunit vaccine is safe and effective in young pigs, induces strong antibody responses, and suppresses viral replication, making it a promising tool for swine disease control and cost-effective vaccination strategies.
Keywords: Classical swine fever; Porcine circovirus type 2; bivalent subunit vaccine; single dose.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





Similar articles
-
A prospective CSFV-PCV2 bivalent vaccine effectively protects against classical swine fever virus and porcine circovirus type 2 dual challenge and prevents horizontal transmission.Vet Res. 2023 Jul 11;54(1):57. doi: 10.1186/s13567-023-01181-x. Vet Res. 2023. PMID: 37434231 Free PMC article.
-
E2-based mRNA vaccine encapsulated in lipid nanoparticles protects pigs against classical swine fever virus.J Virol. 2025 Aug 21:e0097825. doi: 10.1128/jvi.00978-25. Online ahead of print. J Virol. 2025. PMID: 40838721
-
Evaluation of PCV2 vaccine immunogenicity and efficacy using ELISpot to detect virus-specific memory B cells.Porcine Health Manag. 2025 Jul 10;11(1):38. doi: 10.1186/s40813-025-00452-7. Porcine Health Manag. 2025. PMID: 40640918 Free PMC article.
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
Strategies of Classical Swine Fever Immune Evasion.Int J Mol Sci. 2025 Aug 14;26(16):7838. doi: 10.3390/ijms26167838. Int J Mol Sci. 2025. PMID: 40869159 Free PMC article. Review.
References
-
- Fort M., Sibila M., Perez-Martin E., Nofrarias M., Mateu E., Segales J. One dose of a porcine circovirus 2 (PCV2) sub-unit vaccine administered to 3-week-old conventional piglets elicits cell-mediated immunity and significantly reduces PCV2 viremia in an experimental model. Vaccine. 2009;27:4031–4037. doi: 10.1016/j.vaccine.2009.04.028. - DOI - PubMed
LinkOut - more resources
Full Text Sources

